Abstract
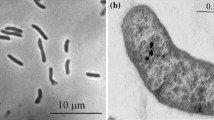
Two deltaproteobacterial sulfate reducers, designated strain I.8.1T and I.9.1T, were isolated from the oxygen minimum zone water column off the coast of Peru at 400 and 500 m water depth. The strains were Gram-negative, vibrio-shaped and motile. Both strains were psychrotolerant, grew optimally at 20°C at pH 7.0–8.0 and at 2.5–3.5% NaCl (w/v). The strains grew by utilizing hydrogen/acetate, C3–4 fatty acids, amino acids and glycerol as electron acceptors for sulfate reduction. Fumarate, lactate and pyruvate supported fermentative growth. Sulfate, sulfite, thiosulfate and taurin supported growth as electron acceptors. Both strains were catalase-positive and highly oxygen-tolerant, surviving 24 days of exposure to atmospheric concentrations. MK6 was the only respiratory quinone. The most prominent cellular fatty acid was iso-17:1-ω9c (18%) for strain I.8.1T and iso-17:0-ω9c (14%) for strain I.9.1T. The G+C contents of their genomic DNA were 45–46 mol%. Phylogenetic analysis of 16S rRNA and dsrAB gene sequences showed that both strains belong to the genus Desulfovibrio. Desulfovibrio acrylicus DSM 10141T and Desulfovibrio marinisediminis JCM 14577T represented their closest validly described relatives with pairwise 16S rRNA gene sequence identities of 98–99%. The level of DNA-DNA hybridization between strains I.8.1T and I.9.1T was 30–38%. The two strains shared 10–26% DNA-DNA relatedness with D. acrylicus. Based on a polyphasic investigation it is proposed that strains I.8.1T and I.9.1T represent a novel species for which the name Desulfovibrio oceani sp. nov. is proposed with the two subspecies D. oceani subsp. oceani (type strain, I.8.1T = DSM 21390T = JCM 15970T) and D. oceani subsp. galateae (type strain, I.9.1T = DSM 21391T = JCM 15971T).

Similar content being viewed by others
Abbreviations
- SRB:
-
Sulfate-reducing bacteria
- dsrAB :
-
Genes (dsrA and dsrB) encoding the alpha and beta subunit of dissimilatory bisulfite reductase
- DSMZ:
-
Deutsche Sammlung von Microorganismen und Zellkulturen (Braunschweig, Germany)
- OMZ:
-
Oxygen minimum zone
References
Bale SJ, Goodman K, Rochelle PA, Marchesi JR, Fry JC, Weightman AJ, Parkes RJ (1997) Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol 47:515–521
Cashion P, Holder-Franklin MA, McCully J, Franklin M (1977) A rapid method for the base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Cowan ST, Steel KJ (1993) Manual for the identification of medical bacteria. In: Barrow GI, Feltham RKA (eds) 3rd edn. Cambridge University Press, Cambridge
Cypionka H (2000) Oxygen respiration by Desulfovibrio species. Annu Rev Microbiol 54:827–848
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation of bacterial DNA. Rur J Biochem 12:133–142
Fuchs BM, Woebken D, Zubkov MV, Burkill P, Amann R (2005) Molecular identification of picoplankton populations in contrasting waters of the Arabian Sea. Aquat Microb Ecol 39:145–157
Fuenzalida R, Schneider W, Garcés-Vargas J, Bravoc L, Lange C (2009) Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep Sea Res II 56:992–1003
Hardy JA, Hamilton WA (1981) The oxygen tolerance of sulfate-reducing bacteria isolated from North Sea waters. Curr Microbiol 6:259–262
Helly JJ, Levin LA (2004) Global distribution of naturally occurring marine hypoxia on continental margins. Deep-Sea Res I 51:1159–1168
Huss VAR, Festl H, Schleifer K-H (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Isaksen MF, Teske A (1996) Desulforhopalus vacuolatus gen. nov., sp. nov., a new moderately psychrophilic sulphate-reducing bacterium with gas vacuoles isolated from temperate estuary. Arch Microbiol 166:160–168
Kjeldsen KU, Loy A, Jakobsen TF, Thomsen TR, Wagner M, Ingvorsen K (2007) Diversity of sulfate-reducing bacteria from an extreme hypersaline sediment, Great Salt Lake (Utah). FEMS Microbiol Ecol 60:287–298
Kjeldsen KU, Jakobsen TF, Glastrup J, Ingvorsen K (2009) Desulfosalsimonas propionicica gen. nov. sp. nov., a novel halophilic sulfate-reducing member of the family Desulfobacteraceae isolated from sediment of Great Salt Lake (Utah). Int J Syst Evol Microbiol (in press)
Klouche N, Basso O, Lascourreges JF, Cayol JL, Thomas P, Fauque G, Fardeau ML, Magot M (2009) Desulfocurvus vexinensis gen. nov., sp. nov., a sulphate-reducing bacterium isolated from a deep subsurface aquifer. Int J Syst Evol Microbiol (in press)
Knoblauch C, Sahm K, Jørgensen BB (1999) Psychrophilic sulfate-reducing bacteria isolated from permanently cold arctic marine sediments: description of Desulfofrigus oceanense gen. nov., sp. nov., Desulfofrigus fragile sp. nov., Desulfofaba gelida gen. nov., sp. nov., Desulfotalea psychrophila gen. nov., sp. nov. and Desulfotalea arctica sp. nov. Int J Syst Bacteriol 49:1631–1643
Kondo R, Butani J (2007) Comparison of the diversity of sulfate-reducing bacterial communities in the water column and the surface sediments of a Japanese meromictic lake. Limnol 8:131–141
Kuever J, Rainey FA, Widdel F (2005) Family I. Desulfovibrionaceae fam. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey’s Manual of Systematic Bacteriology (The Proteobacteria), vol 2, part C (The Alpha-, Beta-, Delta-, and Epsilonproteobacteria), 2nd edn. Springer, New York, pp 926–943
Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, Gutiérrez D, Amann R, Jetten MS, Kuypers MM (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci USA 106:4752–4757
Lobo SAL, Melo AMP, Carita JN, Teixeira M, Saraiva LM (2007) The anaerobe Desulfovibrio desulfuricans ATCC 27774 grows at nearly atmospheric oxygen levels. FEBS Lett 581:433–436
Loy A, Lehner A, Lee N, Adamczyk J, Meier H, Ernst J, Schleifer K-H, Wagner M (2002) Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl Environ Microbiol 68:5064–5081
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar A, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüssmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer K-H (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Pfennig N (1978) Rhodocyclus pupureus gen. nov. and sp. nov., a rind-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Ploug H, Kühl M, Buchholz-Cleven B, Jørgensen BB (1997) Anoxic aggregates–an ephemeral phenomenon in the pelagic environment? Aquat Microb Ecol 13:285–294
Postgate JR, Campbell LL (1966) Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev 30:732–738
Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acid Res 35:7188–7198
Revsbech NP, Larsen LH, Gundersen J, Dalsgaard T, Ulloa O, Thamdrup B (2009) Determination of the ultra-low oxygen concentrations in the oxygen minimum zones by the STOX sensor. Limnol Oceanogr: Methods 7:371–381
Sass H, Cypionka H (2004) Isolation of sulfate-reducing bacteria from the terrestrial deep subsurface and description of Desulfovibrio cavernae sp. nov. Syst Appl Microbiol 27:541–548
Scherer S, Neuhaus K (2007) Life at low temperatures. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Ecophysiology and biochemistry, vol 2, 3rd edn. Springer, New York, pp 210–262
Stevens H, Ulloa O (2008) Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ Microbiol 10:1244–1259
Suzuki D, Ueki A, Amaiski A, Ueki K (2007) Desulfobulbus japonicus sp. nov., a novel Gram-negative propionate-oxidizing, sulphate-reducing bacterium isolated from estuarine sediment in Japan. Int J Syst Evol Microbiol 57:849–855
Takii S, Hanada S, Hase Y, Tamaki H, Uyeno Y, Sekiguchi Y, Matsuura K (2008) Desulfovibrio marinisediminis sp. nov., a novel sulfate-reducing bacterium isolated from coastal marine sediment via enrichment with Casamino acids. Int J Syst Evol Microbiol 58:2433–2438
Tarpgaard IH, Boetius A, Finster K (2005) Desulfobacter psychrotolerans sp. nov., a new psychrotolerant sulfate-reducing bacterium and descriptions of its physiological response to temperature changes. Antonie Van Leeuwenhoek 89:109–124
Teske A, Wawer C, Muyzer G, Ramsing NB (1996) Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol 62:1405–1415
van der Maarel M, van Bergeijk S, van Werkhoven AF, Laverman AM, Meijer WG, Stam WT, Hansen TA (1996) Cleavage of dimethylsulfoniopropionate and reduction of acrylate by Desulfovibrio acrylicus sp. nov. Arch Microbiol 166:109–115
Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J (1996) Polyphasic taxonomy, a consensus approach to bacterial systematics. Microb Rev 60:407–438
Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA (1998) Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180:2975–2982
Wakeham SG, Amann R, Freeman KH, Hopmans EC, Jørgensen BB, Putnam IF, Schouten S, Damste′ JSS, Talbot HM, Woebken D (2007) Microbial ecology of the stratified water column of the Black Sea as revealed by a comprehensive biomarker study. Org Geochem 38:2070–2097
Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Truper HG (1987) Report of the ad hoc committee on the reconciliation of the approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Widdel F, Bak F (1992) Gram-negative mesotrophic sulfate-reducing bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, vol 4, 2nd edn. Springer, New York, pp 3352–3378
Acknowledgments
We thank Tove Wiegers and Trine S. Thomsen for expert technical assistance. We are grateful to the Identification Service of the Deutsche Sammlung von Mikroorganismen und Zellkulturen for performing the DNA-DNA hybridization analyses. The authors gratefully acknowledge the advice given by Professor Euzéby in naming the isolates. The master and the crew onboard the Danish Naval vessel “Vædderen” are thanked for their help and hospitality. Sample collection was carried out during the Galathea 3 expedition under the auspices of the Danish Expedition Foundation. This is Galathea 3 contribution P54. This study was supported by SNF Grant No. 272-05-0363 and Carlsbergfondet 2007010316.
Author information
Authors and Affiliations
Corresponding author
Additional information
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA and the dsrAB gene sequences for strain I.8.1T are FJ655907 and FJ655910; and FJ655908 and FJ655911 for strain I.9.1T. Furthermore, the 16S rRNA gene sequence of Desulfovibrio desulfuricans subsp. aestuarii NCIMB 9335T and the dsrAB sequence of Desulfovibrio acrylicus DSM 10141T were determined in this study and deposited under the GenBank/EMBL/DDBJ accession numbers FJ655909 and FJ655912, respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Finster, K.W., Kjeldsen, K.U. Desulfovibrio oceani subsp. oceani sp. nov., subsp. nov. and Desulfovibrio oceani subsp. galateae subsp. nov., novel sulfate-reducing bacteria isolated from the oxygen minimum zone off the coast of Peru. Antonie van Leeuwenhoek 97, 221–229 (2010). https://doi.org/10.1007/s10482-009-9403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-009-9403-y