Abstract
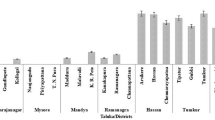
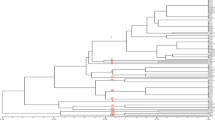
Genetic diversity among 27 isolates (23 from chickpea and 4 from other host crops) of Rhizoctonia bataticola representing 11 different states of India was determined by random amplified polymorphic DNA (RAPD), internal transcribed spacer restriction fragment length polymorphism (ITS-RFLP) and ITS sequencing. The isolates showed variability in virulence test. Unweighted paired group method with arithmetic average cluster analysis was used to group the isolates into distinct clusters. The clusters generated by RAPD grouped all the isolates into six categories at 40% genetic similarity. High level of diversity was observed among the isolates of different as well as same state. Some of the RAPD (OPN 4, OPN 12, and OPN 20) markers clearly distinguished majority of the isolates into the area specific groups. The ITS I, 5.8rDNA and ITS II regions of 11 isolates representing different RAPD groups were amplified with primers ITS 1 and ITS 4 and digested with seven restriction enzymes. The restriction enzymes DraI, MboI, RsaI, and AluI were found to be suitable for differentiating the isolates into five categories by showing isolate specific ITS-RFLP patterns. The isolates were variable in their nucleotide sequences of the ITS regions. This is the first study on genetic diversity among chickpea isolates of R. bataticola.








Similar content being viewed by others
References
Almeida AMR, Abdelnoor RV, Arias CAA, Carvalho VP, Jacoud Filho DS, Marin SRR, Benato LC, Pinto MC, Carvalho CGP (2003) Genotypic diversity among Brazilian isolates of Macrophomina phaseolina revealed by RAPD. Fitopatol Bras 28:279–285
Arabi MIE, Jawhar M (2007) Molecular and pathogenic variation identified among isolates of Cochliobolus sativus. Australas Plant Pathol 36:17–21
Ashby SF (1927) Macrophomina phaseoli (Maubl) Com. Nov. the pycnidial stage of Rhizoctonia bataticola (Taub.) Butler. Trans Br Mycol Soc 12:141–142
Beas-Fernandiz R, De Santiago AD, Hernandez-Delgado S, Mayek-Perez N (2006) Characterization of mexican and non-mexican isolates of Macrophomina phaseolina based on morphological characteristics, pathogenicity on bean seeds and endoglucanase genes. J Plant Pathol 88:53–60
Brinboim HC, Dolly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Briton-Jones HR (1925) Mycological work in Egypt during the period 1920–1922. Egypt Ministry of Agriculture Technical and Science Bulletin, Cairo 49:129
Cobb BD, Clarkson JM (1994) A simple procedure for optimizing the polymerase chain reaction (PCR) using modified Tuguchi methods. Nucleic Acids Res 22:3801–3805
Dubey SC, Singh SR (2008) Virulence analysis and oligonucleotide fingerprinting to detect diversity among Indian isolates of Fusarium oxysporum f. sp. ciceris causing chickpea wilt. Mycopathologia 165:389–406
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research. Wiley, Singapore, pp 139–153
Hall TA (1999) BIOEDIT: a user-friendly biological sequences alignment, editor and analysis of program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Holliday P (1980) Fungus diseases of tropical crops. Cambridge university press, Cambridge, NY10022, p 607
Jana TK, Sharma TR, Prasad RD, Arora DK (2003) Molecular characterization of Macrophomina phaseolina and Fusarium species by single primer RAPD technique. Microbiol Res 158:249–257
Jana TK, Sharma TR, Singh NK (2005) SSR-based detection of genetic variability in the charcoal root rot pathogen Macrophomina phaseolina. Mycol Res 109:81–86
Mayek-Perez N, Lopez-Castaneda C, Gonzalez-Chavira M, Garcia-Espinoa R, Acosta-Gallegos J, Martinez de la Vega OM, Simpson A (2001) Variability of mexican isolates of macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiol Mol Plant Pathol 59:257–264
Mendal M, Higa A (1970) Calcium dependent bacteriophase DNA isolation. J Mol Biol 53:159–162
Murray MG, Thompson WE (1980) RAPD isolation of high molecular weight DNA. Nucleic Acids Res 8:4321–4325
Nene YL, Hawara MP, Reddy MV (1981) Chickpea disease resistance screening technique. ICRISAT Inf Bull 10:4–5
Nene YL, Shiela VK, Sharma SB (1984) A world list of chickpea (Cicer arietinum) and pigeon pea (Cajanus cajan) pathogen. ICRISAT Pulse Pathol Prog Rep 32:19
Pande S, Kumar KK, Rao JN (2004) Evaluation of chickpea lines for resistance to dry root rot caused by Rhizoctonia bataticola. Int Chickpea Pigeonpea Newsl 11:37–38
Pecina-Quintero V, De la Vega O, Alvarado Balleza M, Vandemark G, Williams-Alanis H (2002) Comparacion de dos Sistemas de Marcadores Moleculares en el Analisis de Lasrelaciones Geneticas de Macrophomina phaseolina. Rev Mex Fitopatol 19:72–80
Purkayastha S, Kaur B, Chaudhury D (2006) Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR-based molecular markers. Plant Pathol 55:106
Rajkumar BF, Kuruvinashetti MS (2007) Genetic variability of sorghum charcoal rot pathogen (Macrophomina phaseolina) assessed by random DNA markers. Korean Plant Pathol J 23:45–50
Rohlf JF (2005) NTSYS-pc: numerical taxonomy and multivariate analysis system version 2.2. Exter Software, Setauket, NY
Sambrook JE, Fritsch F, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Su G, Suh SO, Schneider RW, Russian JS (2001) Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology 91:120–126
Van Emden HF, Ball SL, Rao MR (1988) Pest diseases and weed problems in pea, lentil and faba bean and chickpea. In: Summerfield RJ (ed) World crops: cool season food legumes. Kluwer, Dordrecht, pp 519–534
Vandemark G, Martinez O, Pecina V, de Jesus Alvarado M (2000) Assessment of genetic relationships among isolates of Macrophomina phaseolina using a simplified AFLP technique and two different methods of analysis. Mycologia 92:656–664
Vishwadhar, Chaudhary RG (2001) Disease resistance in pulse crop-current status and future approaches. In: Nagarajan S, Singh DP (eds) The role of resistance in intensive agriculture. Kalyani, New Delhi, pp 144–157
White TJ, Bruns T, Lee S, Talyor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninisky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghakhani, M., Dubey, S.C. Determination of genetic diversity among Indian isolates of Rhizoctonia bataticola causing dry root rot of chickpea. Antonie van Leeuwenhoek 96, 607–619 (2009). https://doi.org/10.1007/s10482-009-9375-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-009-9375-y