Abstract
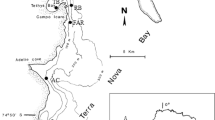
Sponges are well documented to harbor large amounts of microbes. Both culture-dependent and molecular approaches have revealed remarkable bacterial diversity in marine sponges. Fungi are commonly isolated from marine sponges, yet no reports on phylogenetic diversity of sponge-inhabiting fungi exist. In this report, we investigated the phylogenetic diversity of culturable fungi from the Hawaiian alien marine sponges Suberites zeteki and Gelliodes fibrosa. A total of 44 independent isolates were recovered from these two sponge species, representing 7 orders and 22 genera of Ascomycota. The majority (58%) of fungal isolates from S. zeteki resided in the Pleosporales group, while the predominant isolates (52%) from G. fibrosa were members of the Hypocreales group. Though differing in fungal species composition and structure, culturable communities of these two sponges displayed similar phylogenetic diversity. At the genus level, only two genera Penicillium and Trichoderma in the Eurotiales and Hypocreales orders, respectively, were present in both sponge species. The other genera of the fungal isolates were associated with either S. zeteki or G. fibrosa. Some of these fungal genera had been isolated from sponges collected in other marine habitats, but more than half of these genera were identified for the first time in these two marine sponges. Overall, the diversity of culturable fungal communities from these two sponge species is much higher than that observed in studies of marine sponges from other areas. This is the first report of phylogenetic diversity of marine sponge-associated fungi and adds one more dimension to our current understanding of the phylogenetic diversity of sponge-symbiotic microbes.


Similar content being viewed by others
References
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Yalsaceae) from Eucalyptus. Stud Mycol 52:1–142
Atkinson MJ (1987) Rates of phosphorus uptake by coral reef flat communities. Limnol Oceanogr 32:426–435
Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W (2003) Marine invasive alien species: a threat to global biodiversity. Mar Pol 27:313–323
Brasier CM (1997) Fungal species in practice: identifying species units in fungi. In: Claridge M, Dawah H, Wilson M (eds) Species: the units of biodiversity. Capmann & Hall, London, pp 135–170
Bugni TS, Ireland CM (2004) Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep 21:143–163
Burnett J (2003) Fungal populations and species. Oxford University Press, Oxford
Cantin A, Moya P, Miranda MA, Primo J, Primo-Yufera E (1998) Isolation of N-(2-methyl-3-oxodecanoyl)pyrrole and N-(2-methyl-3-oxodec-8-enoyl)pyrrole, two new natural products from Penicillium brevicompactum, and synthesis of analogues with insecticidal and fungicidal activity. J Agric Food Chem 46:4748–4753
Coles SL, DeFelice RC, Eldredge LG, Carlton JT (1999) Historical and recent introductions of non-indigenous marine species into Pearl Harbor, Oahu, Hawaiian Islands. Mar Biol 135:147–158
Coles SL, Eldredge LG (2002) Nonindigenous species introductions on coral reefs: a need for information. Pac Sci 56:191–209
Cutler HG, Cutler SJ, Sayed KE, Dugan FM, Bartlett MG, Hill AA, Hill RA, Parker SR (1999) Koninginin G, A new metabolite from Trichoderma autreoviride. J Nat Prod 62:137–139
De La Camara R, Pinilla I, Munoz E, Buendia B, Steegmann JL, Fernandez-Ranada JM (1996) Penicillium brevicompactum as the cause of a necrotic lung ball in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant 18:1189–1193
Dorner JW (1983) Production of cyclopiazonic acid by Aspergillus tamarii Kita. Appl Environ Microbiol 46:1435–1437
Eldredge LG, Carlton FT (2002) Hawaiian marine bioinvasions: a preliminary assessment. Pac Sci 56:211–212
Friedrich AB, Fischer I, Proksch P, Hacker J, Hentschel U (2001) Temporal variation of the microbial community associated with the Mediterranean sponge Aplysina aerophoba. FEMS Microbiol Ecol 38:105–113
Galstoff PS (1942) Wasting disease causing mortality of sponges in the West Indies and Gulf of Mexico. Proc. 8th Am Sci Congr 3:411–421
Grunig CR, Patrick BC, Duo A, Sieber TN (2007) Suitability of methods for species recognition in the Phialocephala fortinii–Acephala applanata species complex using DNA analysis. Fung Genet Biol DOI 10.1016/j.fgb.2006.12.008
Harrington TC, Rizzo DM (1999) Defining species in the fungi. In: Worrall JJ (ed) Structure and dynamics of fungal populations. Kluwer Press, Dordrecht, The Netherlands
Hawksworth DL (1991) The fungal dimension of biodiversity magnitude significance and conservation. Mycol Res 95:641–655
Hentschel U, Fieseler L, Wehrl A, Gernert C, Steinert M, Hacker J, Horn M (2003) Microbial diversity of marine sponges. In: Müller WEG (ed) Sponges (Porifera). Springer-Verlag Heidelberger, Berlin, Germany, pp 59–88
Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55:167–177
Herndl GL, Weinbauer MG (2003) Marine microbial food web structure and function. In: Wefer G, Lamy F, Mantoura F (eds) Marine science frontiers for Europe. Springer-Verlag, Berlin, pp 265–277
Hill RT (2004) Microbes from marine sponges: a treasure trove of biodiversity for natural products discovery. In: Bull AT (ed) Microbial diversity and bioprospecting. ASM press, Washington, DC, pp 177–190
Hoehnk W, Ulken A (1979) Fungi from marine sponges. Inst Meeresforsch Bremerh 17:199–204
Höller U, König GM, Wright AD (1999) Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J Nat Prod 62:114–118
Höller U, Wright AD, Matthee GF, Konig GM, Draeger S, Aust HJ, Schulz B (2000) Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol Res 104:1354–1365
Hyde KD, Jones EBG, Leano E, Pointing SB, Poonyth AD, Vrijmoed LLP (1998) Role of fungi in marine ecosystems. Biodivers Conserv 7:1147–1161
Hyde KD, Pointing SB (2000) Marine mycology: a practical approach. Fungal Diversity Press, Hong Kong
Imhoff JF, Stoehr R (2003) Sponge-associated bacteria: general overview and special aspects of bacteria associated with Halichondria panicea. In: Müller WEG (ed) Sponges (Porifera). Springer-Verlag Heidelberger, Berlin, Germany, pp 35–57
Jensen PR, Fenical W (2002) Secondary metabolites from marine fungi. In: Hyde KD (ed) Fungi in marine environemnts. Fungal Diversity Press, Hong Kong, pp 293–315
Jones EBG (1995) Ultrastructure and taxonomy of the aquatic ascomycetous order halosphaeriales. Can J Bot 73:S790–S801
Jones EBG, Bremer G (1976) Physiology of the higher marine fungi. In: Jones EBG (ed) Recent advances in aquatic mycology. Paul Elek Ltd., London, UK, pp 260–278
Kimura H, Sato M, Sugiyama C, Naganuma T (2001) Coupling of thraustochytrids and POM, and of bacterio- and phytoplankton in a semi-enclosed coastal area: implication for different substrate preference by the planktonic decomposers. Aquat Microb Ecol 25:293–300
Kohlmeyer J (1984) Tropical marine fungi. Mar Ecol 5:329–378
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology the higher fungi. Academic Press, New York, London
Kohlmeyer J, Volkmann-Kohlmeyer B (1991) Illustrated key to the filamentous higher marine fungi. Bot Mar 34:1–35
Kohlmeyer J, Volkmann-Kohlmeyer B (2003) Mycological research news. Mycol Res 107:385–387
Kohlmeyer J, Volkmann-Kohlmeyer B (1990) New Species of Koralionastes (Ascomycotina) from the Caribbean and Australia. Can J Bot 68:1554–1559
Kohn LM (1995) The clonal dynamic in wild and agricultural plant–pathogen populations. Can J Bot 73:S1231–S1240
König GM, Kehraus S, Seiber SF, Abdel-Lateff A, Muller D (2006) Natural products from marine organism and their associated microbes. Chembiochem 7:229–238
Lee YK, Lee J-H, Lee HK (2001) Microbial symbiosis in marine sponges. J Microbiol 39:254–264
Macias FA, Varela RM, Simonet AM, Cutler HG, Cutler SJ, Ross SA, Dunbar DC, Dugan FM, Hill RA (2000) (+)-Brevione A. The first member of a novel family of bioactive spiroditerpenoids isolated from Penicillium brevicompactum Dierckx. Tetrahedron Lett 41:2683–2686
Magnuson JK, Lasure LL (2002) Fungal diversity in soils as assessed by direct culture and molecular techniques. Paper presented at the 102nd General Meeting of the American Society for Microbiology, Salt Lake City, 19–23 May 2002
Marchisio VF, Giannetta A, Nosenzo C (1993) Penicillium brevicompactum in the home of a patient suffering from bronchial asthma. Allionia 32:57–64
Molitoris HP, Schaumann K (1986) Physiology of marine fungi a screening program for growth and enzyme production. In: Moss ST (ed) The biology of marine fungi. Fourth international marine mycology symposium, Portsmouth, England, August 1985. Cambridge University Press, Cambridge New York, pp 35–48
Morrison-Gardiner S (2002) Dominant fungi from Australian coral reefs. Fung Diver 9:105–121
Nakagawa-Yoshida K, Ando M, Etches RI, Dosman JA (1997) Fatal cases of farmer’s lung in a Canadian family: probable new antigens, Penicillium brevicompactum and P. olivicolor. Chest 111:245–248
Namikoshi M, Akano K, Kobayashi H, Koike Y, Kitazawa A, Rondonuwu AB, Pratasik SB (2002) Distribution of marine filamentous fungi associated with marine sponges in coral reefs of Palau and Bunaken Island, Indonesia. J Tokyo Univ Fish 88:15–20
O’Brien HE, Parrent JL, Jackson JA, Moncalvo J-M, Vilgalys R (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 71:5544–5550
Petersen RH, Hughes KW (1999) Species and speciation in mushrooms: development of a species concept poses difficulties. Bioscience 49:440–452
Proksch P, Ebel R, Edrada RA, Wray V, Steube K (2003) Bioactive natural products from marine invertebrates and associated fungi. In: Müller WEG (ed) Sponges. Springer-Verlag, Heidelberg, Berlin, Germany, pp 117–142
Richter W (1985) Marine sponges as substrate for Thraustochytriaceae marine lower fungi. Inst Meeresforsch Bremerh 20:141–150
Roose-Amsaleg C, Brygoo Y, Harry M (2004) Ascomycete diversity in soil-feeding termite nests and soils from a tropical rainforest. Environ Microbiol 6:462–469
Rovirosa J, Diaz-Marrero A, Darias J, Painemal K, Martin AS (2006) Secondary metabolites from marine Penicillium brevicompactum. J Chin Chem Soc 51:775–778
Sarma AS, Daum T, Muller WEG (1993) Secondary metabolites 549 from marine sponges. Ullstein Mosby, Berlin
Schmechel D, Gorny RL, Simpson JP, Reponen T, Grinshpun SA, Lewis DM (2003) Limitations of monoclonal antibodies for monitoring of fungal aerosols using Penicillium brevicompactum as a model fungus. J Immunol Methods 283:235–245
Sharma BK, Basandrai AK (2000) Effectiveness of some fungicides and biocontrol agents for the management of Karnal bunt of wheat. J Mycol Plant Pathol 30:76–78
Sparks AK (1985) Synopsis of invertebrate pathology: exclusive of insects. Elsevier Science Publisher, New York
Skory CD, Chang P-K, Cary J, Linz JE (1992) Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol 58:3527–3537
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony and other programs, 4.0b10 ed. Sinauer Associates, Sunderland, Mass
Takahasi S, Kinoshita T, Takahashi M (1994) Adenophostins A and B: potent agonists of inositol–1,4,5-trisphosphate receptor produced by Penicillium brevicompactum. Structure elucidation. J Antibiot 47:95–100
Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveira H (2002) Genetic and morphological characterization of Colletotrichum acutatum causing anthracnose of lupins. Phytopathology 92:986–996
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Terashima Y, Igusa H, Ohga S (2002) Influence of contamination by Penicillium brevicompactum and Trichoderma harzianum during Lentinula edodes spawn run on fruiting in sawdust-based substrates. Mycoscience 43:277–280
Vacelet J, Vacelet E, Gaino E, Gallissian MF (1994) Bacterial attach of spongin skeleton during the 1986–1990 Mediterranean sponge disease. In: van Soest RWM, van Kempen TMG, Braekman JC (eds) Sponges in time and space. A. A. Balkema, Rotterdam, pp 355–362
Vasiliauskas R, Lygis V, Thor M, Stenlid J (2004) Impact of biological (Rotstop) and chemical (urea) treatments on fungal community structure in freshly cut Picea abies stumps. Biol Control 31:405–413
Verkley GJM, da Silva M, Wicklow DT, Crous PW (2004) Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies Mycol 50:323–335
Webster N, Hill RT (2001) The culturable microbial community of the Great Barrier Reef sponge Rhopaloeides odorabile is dominated by an α-proteobacterium. Mar Biol 138: 843–851
Wang G (2006) Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol 33:545–551
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application. Academic Press Inc., San Diego, pp 315–322
Acknowledgments
We thank D. Henderson for her many helpful suggestions on improving this manuscript. This work is funded by NOAA grant NA04OAR4600196 and University of Hawaii Sea Grant under institutional grants NA05OAR4171048 and NA16RG2254. The views expressed herein are those of the authors and do not necessarily reflect the views of NOAA or any of its subagencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Li, Q. & Zhu, P. Phylogenetic diversity of culturable fungi associated with the Hawaiian Sponges Suberites zeteki and Gelliodes fibrosa . Antonie van Leeuwenhoek 93, 163–174 (2008). https://doi.org/10.1007/s10482-007-9190-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-007-9190-2