Abstract
In South Africa young women bear a disproportionate burden of HIV infection however, risk factors for HIV acquisition are not fully understood in this setting. In a cohort of 245 women, we used proportional hazard regression analysis to examine the association of demographic, clinical and behavioural characteristics with HIV acquisition. The overall HIV incidence rate (IR) was 7.20 per 100 women years (wy), 95 % confidence interval (CI) 4.50–9.80. Women 18–24 years had the highest HIV incidence (IR 13.20 per 100 wy, 95 % CI 6.59–23.62) and were almost three times more likely to acquire HIV compared to women 25 years and older [adjusted Hazard Ratio (aHR) 2.61, 95 % CI 1.05–6.47]. Similarly, women in relationships with multiple sex partners had more than twice the risk of acquiring HIV when compared to women who had no partner or who had a husband or stable partner (aHR 2.47, 95 % CI 0.98–6.26). HIV prevention programmes must address young women’s vulnerability and sex partner reduction in this setting.
Similar content being viewed by others
Introduction
Into the fourth decade of the human immunodeficiency virus (HIV) epidemic, South Africa has an estimated 6.1 million people living with HIV and contributes 17 % of the global HIV burden, yet accounts for only 0.7 % of the world’s population [1, 2]. With the majority of infections heterosexually acquired, young women bear a disproportionate burden of HIV. Sentinel surveillance among pregnant women shows that in 1990, HIV prevalence was 0.7 % and by 1998 increased exponentially to 22.8 %, peaking at 30.2 % in 2010 [3]. Data from national population based surveys show similar trends with high HIV prevalence in young women compared to young men [4, 5]. HIV prevalence peaked at 36.0 % among women aged 30–34 years and at 28.8 % among men in the 35–39 year-old age group [5]. HIV prevalence in women aged 15–19 years was 5.6 %, eight times higher than males in the same age group, and increased to 17.4 % among women aged 20–24 years and 28.4 % in the 25–29 year age group [5]. Within South Africa there is significant geographical variation in the distribution of HIV and the province of KwaZulu-Natal remains the worst affected. Whilst prevalence of HIV has been useful to understand the evolving epidemic, HIV incidence rate (IR) is a more sensitive measure to monitor the epidemic. Amongst rural and urban women the HIV IRs have been 6.5 and 6.4 per 100 women years (wy) respectively, and in young women <18 years 4.7 per 100 wy [6, 7]. These data underscore the need to understand the risk factors contributing to these high HIV prevalence and IRs.
The key risk factors for HIV in the South African setting include a combination of structural, behavioural and biological factors. Poverty, labour migration, urbanisation, gender inequalities and gender-based violence contribute to an increased susceptibility to HIV [8, 9]. At the individual level, being single, unemployed or not achieving a high school education increase vulnerability and predispose young women to HIV [5, 10, 11]. High risk sexual behaviour such as engaging in multiple concurrent partnerships, transactional sex and age-disparate relationships, has contributed significantly to enhancing HIV risk and is exacerbated by the inability of women to negotiate condom use even in long-term partnerships [9, 12–15]. Physiological changes of the genital tract as well as factors which affect the integrity of the genital tract epithelium such as sexually transmitted infections (STIs) and intra-vaginal insertion practices, either for cleansing or enhancing sex, may increase susceptibility to HIV by facilitating viral entry [9, 16–19]. Recent data suggests that genital tract inflammation in women with symptomatic and asymptomatic STIs may upregulate HIV susceptible target cells at the mucosal level thereby aiding transmission [20]. The association between hormonal contraception use, in particular progestogen-only injectable preparations, and HIV risk remains contentious as current biological and epidemiological evidence is limited [21, 22].
As the HIV epidemic evolves, it is important to understand the factors that contribute to vulnerability and risk so that the design of HIV prevention programmes are tailored taking these into account. In this prospective cohort study we explored factors associated with HIV acquisition in a generalised epidemic setting.
Methods
Study Setting and Study Population
The Centre for the AIDS Programme of Research in South Africa (CAPRISA) initiated the CAPRISA 002 study to advance the understanding of HIV-1 subtype C acquisition, pathogenesis and disease progression. Between August 2004 and May 2005 volunteers from the city of Durban, KwaZulu-Natal, South Africa were recruited for study participation. Eligibility criteria and screening and enrolment procedures have been described [23]. Briefly women 18 years and older, self-identifying as sex workers or having had at least three partners in the 3 months prior to recruitment, and testing HIV negative were eligible for study participation.
Study Procedures
Volunteers were provided with verbal and written information about the study objectives and procedures after which written consent for screening procedures and for long-term storage of specimens were obtained. Women agreeing to participate in the study received pre and post-test counselling for HIV testing and risk reduction counselling and had blood samples collected for HIV antibody testing. Women testing HIV positive were referred to support services for ongoing care and psychosocial support. All women testing HIV antibody negative or indeterminate were enrolled until the study endpoint of HIV infection or for a period of 24 months.
At baseline, trained nurses administered questionnaires to all participants to obtain information regarding socio-demographic history, risk behaviour, knowledge about HIV and more direct questions related to sex work. A complete physical examination was undertaken at baseline and at each monthly follow up visit. Urine pregnancy testing was done if clinically indicated or upon request by a participant [23]. Pelvic examination for genital sample collection was undertaken at baseline and at 6 monthly intervals. Blood specimen collection was done at baseline (safety monitoring tests, serological testing for STIs and long-term storage), at each monthly visit (HIV testing) and at each six-monthly visit (safety monitoring tests and serological testing for STIs).
Laboratory Evaluations
HIV testing was done using two rapid antibody tests using the Determine HIV-1 test (Abbott Diagnostics, Johannesburg, South Africa) and the Capillus HIV-1/HIV-2 test (Trinity Biotech, USA) followed by HIV-1 RNA polymerase chain reaction (PCR) testing using the Cobas AmpliScreen Multiprep HIV-1 test version 1.5 and the Cobas AmpliPrep/Cobas Amplicor HIV-1 Monitor test version 1.5 (Roche Diagnostics, Branchburg, New Jersey, USA) if at least one rapid antibody test was negative [24]. Confirmatory HIV ELISA testing was done on PCR positive samples (Enzynost anti HIV1/2 plus Dade Behring, Deerfield, IL, USA) [24]. HIV infection as endpoint was based on a positive HIV-1 antibody test with a previously documented negative HIV-1 antibody test; or the presence of HIV-1 RNA in the absence of HIV antibodies [23].
Safety monitoring tests comprised haematological and biochemical evaluations. Genital specimens were tested for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium and herpes simplex virus (HSV) type 2 using PCR and bacterial vaginosis (BV) was diagnosed on Gram-stained smears using Nugent’s criteria [20]. Syphilis [Becton-Dickinson Macro-Vue RPR (rapid plasma reagin) card test, BD Diagnostic systems United States and Omega IMMUTREP TPHA (Treponema pallidum Hemaglutination Assay) test, Omega Diagnostics Group PLC], HSV infection (HerpeSelect-1 and HerpeSelect-2, Enzyme-linked immunosorbent assay, Focus Diagnostics, Cypress, CA) and hepatitis B (HBV) infection (Centaur XP, ADVIA Centaur XP Immunoassay System, SIEMENS AG, Germany) were diagnosed serologically.
Data Management
Demographic, behavioural, clinical and laboratory data were captured onto standardised case report forms (CRFs) which were coded with a participant identification number in order to maintain participant confidentiality. CRFs were faxed using the DataFax system (Clinical DataFax Sytems Inc., ON, Canada), with all data verified by data encoders for quality checks and stored in a secure study specific database.
Measures
HIV-1 infection was the main outcome measure. Baseline information on socio- demographic factors (age, number of dependents, educational level), behavioural factors (age at sexual debut, relationship status, frequency and type of sex acts, male and or female condom use (defined as condom use) at last sex act, contraception type, douching, alcohol and substance use prior to sex), sex for compensation (time period involved in sex work, age started sex work, days per week engaged in sex work, number of sites worked at, number of sex clients, short sessions or overnight stays per week with clients and condom use), clinical factors, including pregnancy and laboratory measures (full blood counts, liver function tests, electrolytes, vitamin B12, folate, iron, glucose, calcium, phosphate, CD4+, CD3+ and CD8+ cell counts and STIs), and knowledge questions on HIV prevention and transmission were obtained.
Statistical Analysis
Baseline data were summarised using descriptive statistics, with continuous variables reported as means and standard deviations (SD) or medians and interquartile ranges (IQR), while categorical variables are reported as percentages and actual numbers. Unadjusted and adjusted proportional hazards regression analysis was performed to assess the impact of socio-demographic factors, risk behaviour, sex for compensation and clinical factors on HIV acquisition. Estimated time of HIV infection was defined as the midpoint between the last negative HIV ELISA and the first positive HIV ELISA or 14 days prior to a positive HIV-1 RNA test if the HIV ELISA is negative on the same day [23]. Time to HIV infection was calculated from the date of enrolment until the estimated time of HIV infection. Participants who remained HIV negative were censored at their last visit. Factors with a p value of less than 0.2 in the unadjusted analysis were included in the adjusted model. Given the biological plausibility of anal sex and the risk for HIV acquisition, this variable was included in a multivariable model as was contraception type although current evidence is limited [25–28]. Although some variables relating to sex work had a p value of less than 0.2 in the unadjusted analysis, these were not included in the adjusted model, because a large proportion of the cohort did not identify themselves as sex workers. The second multivariable model was restricted to women reporting sex for compensation.
P values less than 0.05 were considered statistically significant. Analysis was performed using SAS software version 9.3 (SAS Institute Inc., Cary).
Ethics Approval
Ethics approval for the CAPRISA 002 study was obtained from the Universities of Natal (E013/04), Cape Town (025/2004) and Witwatersrand (MM040202). The ethical approval for the secondary analysis of data was granted from the Biomedical Research Ethics Committee, University of KwaZulu-Natal (BE 092/11) and permission to review data stored in the CAPRISA 002 database was approved through the CAPRISA data sharing policy.
Results
A total of 775 women were screened of whom 509 (65.7 %) were ineligible for the following reasons: tested HIV positive (n = 462; 59.6 %), reported less than three sexual partners in the previous 3 months (n = 22; 2.8 %), were pregnant (n = 16; 2.1 %), planned to relocate (n = 4; 0.5 %), younger than 18 years (n = 3; 0.4 %) or afraid of testing procedures (n = 2; 0.3 %) [23]. Twenty-one (2.7 %) women were eligible for study participation but did not return for enrolment and 245 (31.6 %) were enrolled into the study.
After 390 wy of follow up, 28 women acquired HIV, yielding an IR of 7.20 per 100 wy [95 % confidence interval (CI) 4.50–9.80]. Women aged 18–24 years had the highest HIV incidence (IR 13.20 per 100 wy, 95 % CI 6.59–23.62) and among women 25 years and older it was 4.58 per 100 wy (95 % CI 2.50–7.68). Three women were excluded from any risk factor analyses as they were in the window period of HIV infection at study entry.
The baseline characteristics overall and of those acquiring HIV are shown in Table 1. The mean age of 242 women was 34.3 years (SD 10.47, range 18–58), 185 (76.8 %) women had one or more dependents and 156 women (64.5 %) had an educational level which was above grade 8. The mean age at sexual debut was 17 years (SD 2.38, range 12–26). Although a large proportion (191; 78.9 %) self-identified themselves as sex workers, having spent a median of 3 years (IQR 1.3–8.3) in sex work, only 137 (56.6 %) women reported being in a relationship with multiple partners at the time of the study.
Women who acquired HIV were approximately 5 years younger than those who remained HIV negative [t statistic (t) = 2.20, p = 0.029], reported being in relationships with multiple partners more frequently [Fisher’s exact test hypergeometric probability (Fprob) = 0.021, p = 0.054] and had higher baseline serum vitamin B12 levels (t = −2.00, p = 0.047).
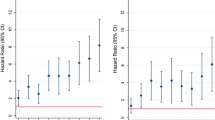
Table 2 shows the socio-demographic, behavioural and biological variables associated with risk of HIV acquisition overall and by sex work. Younger women, aged 18–24 years were almost three times more likely to acquire HIV compared to women 25 years and older [hazard ratio (HR) 2.85, 95 % CI 1.29–6.28; Chi square test statistic (χ2) = 6.73; p = 0.010] and similarly women reporting many partners were at almost three times greater risk of HIV acquisition (HR 2.61, 95 % CI 1.04–6.52; χ2 = 4.18; p = 0.041). A higher educational level, above grade 8, was weakly associated with increased risk for HIV (HR 2.45, 95 % CI 0.92–6.52; χ2 = 3.21; p = 0.073), but no significant associations were found for HIV risk and engaging in anal sex (HR 1.49, 95 % CI 0.68–3.29; χ2 = 0.99; p = 0.321) or age at sexual debut (HR 0.88, 95 % CI 0.73–1.06; χ2 = 1.88; p = 0.170).
Among women reporting sex for compensation, HIV risk remained threefold higher among 18–24 year-old women (HR 3.27, 95 % CI 1.29–8.30; χ2 = 6.24; p = 0.013) and tenfold higher among those reporting many partners (HR 10.32, 95 % CI 1.37–77.55; χ2 = 5.15; p = 0.023). Weak associations were found between increased HIV risk and every additional overnight stay per week with clients (HR 1.33, 95 % CI 0.94–1.88; χ2 = 2.61; p = 0.106) and reduced risk for every additional year spent in sex work (HR 0.93, 95 % CI 0.85–1.02; χ2 = 2.34; p = 0.126).
In the final multivariable model, young age remained significant for HIV risk (HR 2.61, 95 % CI 1.05–6.47; χ2 = 4.27; p = 0.039).
Discussion
In this study young age and having multiple sex partners were associated with risk for HIV acquisition. Despite the introduction of HIV prevention and treatment programmes, the overall HIV IR of 7.20 per 100 wy, and 13.20 per 100 wy among women aged 18–24 years remains unacceptably high [23]. South Africa and many southern African countries continue to experience similar high HIV IRs. Longitudinal studies among women in South Africa have shown HIV IRs of 6.6, 8.5 and 6.5 per 100 wy particularly amongst young women in the province of KwaZulu-Natal [6, 10, 29]. Among pregnant women from the same region, HIV incidence was 10.7 per 100 wy [30]. In Malawi and Zimbabwe, among women recruited from postnatal or family planning clinics, the HIV IRs were 4.20, 4.86 and 4.78 per 100 wy in Lilongwe, Blantyre and Harare respectively [31]. Overall, the highest IR of 5.78 per 100 wy was among young women <25 years, whilst in Rwanda, among antenatal clinic attendees HIV incidence was 10.5 per 100 wy among women <20 years [31, 32]. These studies demonstrate young women’s vulnerability and greater risk of acquiring HIV compared to older women. Our study shows that young age carries a threefold greater risk of HIV acquisition.
The importance of measuring HIV incidence is key to understanding the dynamics of HIV disease to shape and modify effective responses. The persistence of high HIV IRs and the vulnerability of young women is incompletely elucidated in this setting. Recent studies have shown that residing in urban informal settlements, being unmarried or unemployed was associated with higher HIV incidence highlighting the underlying structural and social factors driving the epidemic [5, 10]. Although common in this setting, high risk behaviours such as engaging in age-disparate relationships, was not found to predict HIV risk in a population-based study and is less likely to contribute to high HIV incidence in young women [33]. STIs are known to contribute to HIV risk in women in this region and ongoing research into the immunology of the female genital tract may provide further insight into the biological susceptibility to HIV infection, particularly in young women [10, 20].
Although the vulnerability of young women is well recognised in this setting, risk for HIV is often underestimated since the perceived risk of HIV remains low [5]. South Africa’s epidemic is generalised with HIV prevalence at an unprecedented high level, in excess of 15 % in the adult population, and new infection rates around 2 % per year [5, 34]. However, the majority of HIV-infected individuals are unaware of their HIV status, and this remains a barrier for both treatment access and prevention and helps sustain the epidemic. Poor knowledge of HIV transmission and limited access to and availability of health care services further promotes risk [5].
Our study demonstrates that women in relationships with multiple partners were more likely to acquire HIV. While multiple concurrent partnerships is recognised as a key driver of the epidemic in the Southern African region, the total number of lifetime partners has been found to be a significant predictor of HIV risk [12, 35]. A meta-analysis of 68 studies from sub-Saharan Africa assessing risk factors for HIV acquisition reported that the number of lifetime partners increased the risk of HIV by almost fourfold [odds ratio (OR) 3.64, 95 % CI 2.87–4.62] [35]. In an urban mining community in Carletonville risk for HIV was almost fivefold higher in women reporting two or more lifetime partners (OR 4.88, 95 % CI 3.01–7.89) [36]. This risk was almost double for women reporting more than one partner 3 months prior to enrolment into HIV prevention intervention studies (adjusted OR 1.78, 95 % CI 1.11–2.85) and similar for young women 15–24 years (OR 2.0, 95 % CI 1.1–3.7) participating in the national household survey of South African youth, reporting more than one lifetime partner [4, 37].
In this setting unemployment levels remain high and resources are constrained [9, 38]. Marriage is also uncommon in this setting, leading to instability of relationships [9]. Transactional sex, which may range from serial monogamous relationships to occasional exchange of sex for money or goods, or to employment as a sex worker, provides a means of survival for women and their dependents, resulting in women engaging in multiple partnerships, either sequential or concurrent, in order to meet their basic needs [8, 9]. Hence, emphasis and efforts on monogamy and multiple partner reduction cannot stand alone. The integration of structural approaches into current HIV policy, which address the socio-economic needs of women; and which create an environment of equal opportunities and rights, is crucial if behavioural and biomedical prevention efforts are to succeed. Nonetheless, rigorously including HIV counselling and testing (HCT) across all levels is important and key to knowledge of HIV status. HCT is especially important to help people learn their own and their partners’ status so as to reduce HIV risk. Furthermore, repeat HCT must be promoted in hyperendemic regions.
The use of hormonal contraception was not found to be associated with HIV acquisition. Results from several studies exploring the use of hormonal contraception increasing the risk of HIV acquisition remain inconsistent [21, 22, 27, 39]. Major limitations of these studies have been the failure to test the hypotheses through robust study designs. Earlier studies showed some signals of an association between hormonal contraception and HIV acquisition [27]. Similarly intra-vaginal insertion practices and pregnancy were not associated with HIV risk, though the outcome may be limited by the small sample size.
Whilst elevated serum vitamin B12 levels was associated with HIV acquisition, its causal role requires further exploration. Recent evidence from critically ill elderly medical patients suggests that elevated serum vitamin B12 levels are associated with increased mortality [40, 41]. Although increased levels have been associated with systemic inflammatory markers, its role in mediating HIV risk remains unclear [40]. While genital tract inflammation associated with HIV risk in this cohort has been reported, the role of vitamin B12 as a marker of systemic inflammation and HIV risk requires further evaluation [20].
The major strength of the study was the prospective cohort study design which allowed assessment of risk factors prior to HIV acquisition, however, there are several limitations as well. Firstly, participants had monthly study visits with risk reduction counselling, male and female condom provision, HIV testing with interviewer administered questionnaires; it is therefore possible that participants could have provided socially desirable responses given the sensitive nature of the sexual risk behaviour questions which could have biased the associations. Furthermore, given the wide age range of this cohort (18–58 years), it was difficult to adequately assess some risk behaviours which may be age dependent. Ideally, it would be important to follow up behaviour in those women who remained HIV negative to assess HIV risk over time. Secondly, the small sample size of the study is an important limitation, impacting on the precision of the study findings, therefore the particularly large confidences intervals around some of the point estimates. This may have also limited our ability to demonstrate stronger associations with some variables. Thirdly, the recruitment of high risk women limits the generalizability of the study findings as these may not be representative of women from elsewhere in the province. Finally, as the study excluded women younger than 18 years, it was not possible to measure HIV incidence and risk factors in this young age group in whom HIV incidence is high [7]. Whilst there are ethical challenges in conducting research in adolescents, it is important that young people, especially girls are included into research studies in order to better understand the high rates of HIV acquisition [7, 42].
In conclusion, the findings of this study confirm that young women continue to bear the brunt of the HIV epidemic in this region. It is important that as transmission dynamics change over time, especially in hyperendemic settings, investments towards large-scale, fundamental changes in behaviour, social practices and community norms, address young women’s vulnerability to HIV.
References
UNAIDS. UNAIDS Report on the Global HIV Epidemic 2013. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed April 22, 2014.
Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–33.
National Department of Health. The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey, South Africa. http://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf. Accessed July 14, 2014.
Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19(14):1525–34.
Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, Labadarios D, Onoya D, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press; 2014.
Abdool Karim Q, Kharsany ABM, Frohlich JA, Werner L, Mashego M, Mlotshwa M, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal South Africa. Int J Epidemiol. 2011;40(4):922–30.
Abdool Karim Q, Kharsany AB, Frohlich JA, Werner L, Mlotshwa M, Madlala BT, et al. HIV incidence in young girls in KwaZulu-Natal, South Africa-public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 2012;16(7):1870–6.
Kelly K, Mkhwanazi N, Nkhwashu N, Rapiti R, Mashale R. (2012) HIV prevention situation analysis in KwaZulu-Natal, Mpumalanga and Gauteng provinces, South Africa. USAID Sexual HIV Prevention Programme in South Africa (SHIPP). http://futuresgroup.com/files/publications/Synthesis_of_Research_on_Prevention_of_Sexual_Transmission_of_HIV_in_SA.pdf. Accessed March 20, 2013.
Abdool Karim Q, Sibeko S, Baxter C. Preventing HIV infection in women: a global health imperative. Clin Infect Dis. 2010;50(Suppl 3):S122–9.
Wand H, Ramjee G. Combined impact of sexual risk behaviors for HIV seroconversion among women in Durban, South Africa: implications for prevention policy and planning. AIDS Behav. 2011;15(2):479–86.
Pettifor AE, Levandowski BA, MacPhail C, Padian NS, Cohen MS, Rees HV. Keep them in school: the importance of education as a protective factor against HIV infection among young South African women. Int J Epidemiol. 2008;37(6):1266–73.
Halperin DT. Why is HIV prevalence so severe in Southern Africa? South Afr J HIV Med. 2007;8(1):19–25.
Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25.
Chimbindi NZ, McGrath N, Herbst K, San Tint K, Newell ML. Socio-demographic determinants of condom use among sexually active young adults in rural KwaZulu-Natal, South Africa. Open AIDS J. 2010;4:88–95.
van Loggerenberg F, Dieter AA, Sobieszczyk ME, Werner L, Grobler A, Mlisana K. HIV prevention in high-risk women in South Africa: condom use and the need for change. PLoS One. 2012;7(2):e30669.
Salvana EMT. HIV and STIs: interactions in resource-limited settings. http://www.medscape.com/viewarticle/754637. Accessed March 12, 2014.
McClelland RS, Lavreys L, Hassan WM, Mandaliya K, Ndinya-Achola JO, Baeten JM. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS. 2006;20(2):269–73.
Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8.
Myer L, Wright TC Jr, Denny L, Kuhn L. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Transm Dis. 2006;33(11):683–7.
Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206(1):6–14.
Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808.
WHO. Hormonal contraception and HIV Technical statement. http://whqlibdoc.who.int/hq/2012/WHO_RHR_12.08_eng.pdf. Accessed July 18, 2014.
van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3(4):e1954.
Abdool Karim SS, Mlisana K, Kharsany A, Williamson C, Baxter C, Karim Q. Utilizing nucleic acid amplification to identify acute HIV infection. AIDS. 2007;21(5):653–5.
Kingsley LA, Detels R, Kaslow R, Polk BF, Rinaldo CR Jr, Chmiel J, et al. Risk factors for seroconversion to human immunodeficiency virus among male homosexuals. Results from the Multicenter AIDS Cohort Study. Lancet. 1987;1(8529):345–9.
Abdool Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88(8):1265–6.
Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26.
Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31(1):79–97.
Ramjee G, Kapiga S, Weiss S, Peterson L, Leburg C, Kelly C, et al. The value of site preparedness studies for future implementation of phase 2/IIb/III HIV prevention trials: experience from the HPTN 055 study. J Acquir Immune Defic Syndr. 2008;47(1):93–100.
Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–9.
Kumwenda N, Hoffman I, Chirenje M, Kelly C, Coletti A, Ristow A, et al. HIV incidence among women of reproductive age in Malawi and Zimbabwe. Sex Transm Dis. 2006;33(11):646–51.
Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A. Incident HIV-1 infection in a cohort of young women in Butare. Rwanda. AIDS. 1994;8(11):1585–91.
Harling G, Newell ML, Tanser F, Kawachi I, Subramanian SV, Barnighausen T. Do age-disparate relationships drive hiv incidence in young women? Evidence from a population cohort in rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2014;66(4):443–51.
Statistics South Africa. Mid-year population estimates 2014. http://beta2.statssa.gov.za/publications/P0302/P03022014.pdf. Accessed August 04, 2014.
Chen L, Jha P, Stirling B, Sgaier SK, Daid T, Kaul R, et al. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One. 2007;2(10):e1001.
Zuma K, Gouws E, Williams B, Lurie M. Risk factors for HIV infection among women in Carletonville, South Africa: migration, demography and sexually transmitted diseases. Int J STD AIDS. 2003;14(12):814–7.
Mavedzenge SN, Weiss HA, Montgomery ET, Blanchard K, de Bruyn G, Ramjee G, et al. Determinants of differential HIV incidence among women in three southern African locations. J Acquir Immune Defic Syndr. 2011;58(1):89–99.
Statistics South Africa. http://beta2.statssa.gov.za/. Accessed September 07, 2014.
Morrison CS, Skoler-Karpoff S, Kwok C, Chen PL, van de Wijgert J, Gehret-Plagianos M, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS. 2012;26(4):497–504.
Sviri S, Khalaila R, Daher S, Bayya A, Linton DM, Stav I, et al. Increased Vitamin B12 levels are associated with mortality in critically ill medical patients. Clin Nutr. 2012;31(1):53–9.
Salles N, Herrmann F, Sakbani K, Rapin CH, Sieber C. High vitamin B12 level: a strong predictor of mortality in elderly inpatients. J Am Geriatr Soc. 2005;53(5):917–8.
Nelson RM, Lewis LL, Struble K, Wood SF. Ethical and regulatory considerations for the inclusion of adolescents in HIV biomedical prevention research. J Acquir Immune Defic Syndr. 2010;54(Suppl 1):S18–24.
Acknowledgments
This work was supported by grants from the Comprehensive International Program of Research on AIDS (CIPRA) of the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH), US Department of Health and Human Services (DHHS) [Grant number 5U19 AI051794] and the National Research Foundation, South Africa [Grant number UID 67385]. NN was partially sponsored by the University of KwaZulu-Natal for career development.The authors would like to acknowledge the CAPRISA 002 Study Team and the participants of the CAPRISA 002 Study without whom this work would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naicker, N., Kharsany, A.B.M., Werner, L. et al. Risk Factors for HIV Acquisition in High Risk Women in a Generalised Epidemic Setting. AIDS Behav 19, 1305–1316 (2015). https://doi.org/10.1007/s10461-015-1002-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-015-1002-5