Abstract
Needle-syringe programs (NSP) have been effective in reducing HIV and hepatitis C (HCV) infection among people who inject drugs (PWID). Achieving sustainable reductions in these blood-borne infections requires addressing structural factors so PWID can legally access NSP services. Systematic literature searches collected information on NSP coverage and changes in HIV or HCV infection prevalence or incidence at the population level. Included studies had to document biomarkers (HIV or HCV) coupled with structural-level NSP, defined by a minimum 50 % coverage of PWID and distribution of 10 or more needles/syringe per PWID per year. Fifteen studies reported structural-level NSP and changes in HIV or HCV infection prevalence/incidence. Nine reported decreases in HIV prevalence, six in HCV infection prevalence, and three reported decreases in HIV incidence. The results support NSP as a structural-level intervention to reduce population-level infection and implementation of NSP for prevention and treatment of HIV and HCV infection.
Similar content being viewed by others
Introduction
Injection drug use is one of the most efficient modes of transmission of human immunodeficiency virus (HIV), hepatitis C (HCV), and other blood-borne diseases [1]. Through the multi-person use or “sharing” of injection equipment and drug supplies, people who inject drugs (PWID) represent a key high-risk group for blood-borne virus transmission. There are an estimated 16 million PWID worldwide, of which approximately 3 million are estimated to be HIV-positive [2]. PWID are also at high risk of HCV infection with prevalence among PWID as high as 90 % in some locations [3].
NSP have been implemented in cities, regions and countries worldwide to address HIV and HCV infection among PWID. They have been shown to be beneficial in reducing risky injection behaviors and factors that influence virus transmission [4]. Sterile needles have also been made available through pharmacies for drug users to purchase at low or no cost [5, 6].
Several countries have been distributing needles/syringes for decades, with positive outcomes among PWID. In New Zealand, needles/syringes have been distributed through over 170 community pharmacies, and were an important part of New Zealand’s harm reduction response to problem drug use and the prevention of the spread of blood-borne viruses [7]. In Connecticut, because of the changes in law in 1992, that allowed for legal sales of needles/syringes in pharmacies without prescriptions, resulted in 90 % increase in needles/syringe sales [8]. In addition, syringe-sharing among PWID decreased significantly [9]. In Switzerland, adoption of NSP took place in the early 1990s, and studies reported that those PWID that began their injecting career during the era of NSP had 80 % lower HIV prevalence, HCV infection prevalence, and other blood-borne infections when compared to PWID who began injecting before NSP or during the period of NSP pilot programs [10].
In addition to the country examples noted above, reviews examining NSP have shown promising results in terms of reductions in blood-borne infections and risky injection behaviors [4, 11], predominately focused on small-scale or pilot NSP. Very few of the studies reviewed were conducted in locations where NSP have reached a population-level of needle/syringe coverage among PWID populations (defined operationally by availability of 10 or more needles/syringes per PWID per year coupled with coverage of at least 50 % of the PWID populations).
Achieving long-term and sustainable reduction in HIV and HCV infection requires addressing factors external to the individual PWID, such as laws and regulations, policies or other environmental adaptations that are focused at the larger population level. Interventions that address these external factors are considered structural. Structural interventions have been defined as prevention interventions that include physical, social, cultural, organizational, community, economic, legal and policy factors and promote or impede health by altering the structural context within which health is produced or reproduced [12, 13]. Structural interventions focus on contextual or environmental factors that influence risk behavior, rather than characteristics of individuals who engage in risk behaviors [13, 14]. Research has highlighted the role of structural factors that either facilitate behavior change or act as barriers to risk reduction [12–15], and directly or indirectly affect a person’s ability to reduce the risk of acquiring infection or transmitting infection to others.
Provision of sterile injecting equipment to PWID may require changes in existing policy or paraphernalia laws so that syringes can be purchased at local pharmacies without prescription, drug users can legally possess needles and syringes, including those containing drug residues, and large scale public funding can be used to support NSP. Large-scale implementation of NSP either through changes in policy or use of public funds is structural in nature as these are external to the individual, and theoretically reach sufficient numbers of PWID within a community, such that decreases in morbidity and mortality can be achieved.
While other reviews have looked at access to sterile injecting equipment, most have focused on interventions that examined individual level outcomes, such as changes in individual risk behaviors [4, 16, 17]. The focus on individual-level outcomes highlights a number of biases and limitations in interpretation. First, there may be a strong self-selection effect for high-risk subjects to participate in NSP, making it difficult to compare them with injectors who do not participate in the programs. Second, simply because someone accesses a NSP does not mean that he or she is obtaining a sufficient supply of sterile syringes. This would be particularly likely if the exchange places limits on the numbers of syringes that can be exchanged per visit. Third, focusing on individuals ignores the possibility that a sufficiently large NSP may blur differences between participants in the NSP (who actually attend the program) and other injectors who do not personally attend the NSP but may directly or indirectly obtain supplies of sterile injection equipment from persons who do attend the program through secondary exchange [18].
This review focuses on structural-level intervention studies that examined population-level changes in HIV and HCV infection prevalence and incidence in relation to interventions that attempt to change or modify the social, legal, economic, political or physical environments that shape or constrain injecting drug use behaviors by increasing the availability of sterile syringes and injection equipment to a level that allows for 10 or more syringes per PWID per year with at least 50 % of the PWID population covered by NSP servicesFootnote 1 [19]. For the purpose of this review, structural interventions are defined as those interventions where changes in policy and legal environment have facilitated an increased availability of sterile syringes which subsequently led to population-level changes in HIV and/or HCV prevalence and/or incidence. Although PWID are at high risk for hepatitis B virus (HBV) infection, we excluded HBV outcomes from our analysis because historically there is transmission of HBV at high levels perinatally in locations in Asia, so it would be difficult to link infection to injection in many of the positive persons. Additionally, since there is a vaccine for Hepatitis B, there could be an effect seen that is confounded by those who were vaccinated against HBV; if increases in rates of immunizations are seen, it could decrease the levels of HBV in the population without needle exchange having any direct causal link.
Methods
We conducted a systematic search utilizing Cochrane collaboration methods [20] to identify evidence for structural-level interventions and changes in HIV and HCV infection among PWID. Studies included in the review had to document one of the following: increased access to sterile syringes through pharmacy sales, increased access through large scale NSP, or elimination of laws that prohibit possession of injection equipment. Additionally, there had to be documentation of NSP implementation on a public health scale, with coverage levels reaching at least 10 needles/syringes per PWID and at least 50 % coverage of the PWID population by the NSP. We excluded “pilot program” interventions that distribute fewer than 10 syringes per PWID per yearFootnote 2 [21].
Inclusion Criteria: Types of Studies
A wide range of randomized and non-randomized study designs were eligible for inclusion in this review. These included before-after studies, case–control studies, cohort studies, randomized controlled trials, serial cross-sectional studies, and time series cross-sectional studies. Studies that were considered for inclusion had to contain a quantitative comparison of individuals or groups who received the intervention versus those who did not, or a comparison of individuals or groups before and after receiving the intervention.
Inclusion Criteria: Types of Participants, Intervention, and Outcomes
We included all adult communities (individuals over 18 years of age) of PWID in locations where NSP was in the process of being implemented or greatly expanded. The expansion may be preceded by laws or policies that allowed for legal needle/syringe distribution to at-risk drug users. In addition, we also included studies that documented changes in HIV and HCV infection in relation to expansion of needle/syringe distribution in pharmacy settings.
PWID in the included studies were recruited from many different locations including street locations known to be gathering locations for PWID, drug treatment programs, NSP, and other treatment settings where PWID could be evaluated for HIV or HCV infection through serologic testing.
To be included in this review all studies had to utilize laboratory testing for HIV and/or HCV infection such as changes in HIV sero-incidence or sero-conversion, HCV sero-incidence or sero-conversion, HIV or HCV sero-prevalence, or HIV/HCV co-infection. Those studies that had self-report measurements of blood-borne infection, or did not report outcome data in conjunction with the structural intervention or expansion of the structural intervention were excluded from the review.
Search Strategy
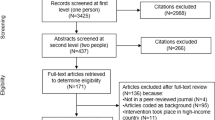
We formulated a comprehensive and exhaustive search strategy utilizing Cochrane collaboration methods [20] in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress) (Fig. 1). The searches were performed without restrictions to setting and were limited to human studies published from January 1, 1980 through March 15, 2011.
We searched multiple databases including the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Literatura Latino Americana e do Caribe em Ciências da Saúde (LILACS), PsycINFO, PubMed, and the Web of Science/Web of Social Science. In addition we searched the Aegis archive of HIV/AIDS conference abstracts [22], which includes the British HIV/AIDS Association, 2001–2008, Conference on Retroviruses and Opportunistic Infections (CROI), 1994–2008, and the European AIDS Society Conference, 2001 and 2003. We searched several conference abstract databases including the International AIDS Society, the Conference on HIV Pathogenesis, Treatment and Prevention (IAS), 2001–2005, International AIDS Conference (IAC), 1985–2006 and the US National HIV Prevention Conference (1999, 2003, and 2005). We also searched the CROI and International AIDS Society web sites for abstracts presented at conferences subsequent to those listed above (CROI, 2009–2010; IAC, 2006–2010; IAS, 2007–2009).
Data Extraction and Management
Data were abstracted independently by four reviewers using a pre-designed, standardized data abstraction form. The reviewers were not blinded to the names of the trial investigators, their institutions or journals of publication. The data abstraction form included information on study, report author(s), year of publication, year in which study was conducted, details of other relevant papers cited, study design, description of intervention and comparison group, country/location of study, sample size, demographics of the study sample, details of outcomes, and study authors’ conclusions.
The team members reviewed and compared extracted data. Differences in data extraction or interpretation of studies were resolved by discussion and consensus, with input from a third member of the review team when necessary, and were discussed on conference calls, as noted below in the “Selection of Studies” section. The final version of the coded data for each study was then entered into Microsoft Excel to facilitate comparisons across studies.
Assessment of Risk of Bias in Included Studies
We used the Cochrane Collaboration’s tool for assessing risk of bias for individual studies [20]. The tool assesses bias across six categories: sequence generation, allocation concealment, blinding of study participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. As none of our included studies were randomized controlled trials (as it would be unethical to withhold NSP from PWID, given previous research), the categories for randomization and blinding usually were not applicable.
Study Rigor
We assessed study rigor on a 9-point scale, with minimum score (low rigor) of 1 and maximum score (high rigor) of 9. Studies received one point for meeting each of the following criteria:
-
Study design includes pre/post intervention data
-
Study design includes control or comparison group
-
Study design includes cohort
-
Comparison groups equivalent at baseline on socio-demographics
-
Comparison groups equivalent at baseline on outcome measures
-
Random assignment (group or individual) to the intervention
-
Participants randomly selected for assessment
-
Control for potential confounders
-
Follow-up rate of 80 % or more
This scale was based on the 8-point rigor assessment scale for systematic reviews of HIV behavioral interventions designed by the Johns Hopkins WHO Synthesizing Intervention Effectiveness Project [23, 24], with an additional item on control for potential confounders.
Data Analysis
Abstracts of all studies identified by electronic or bibliographic scanning were examined by four reviewers who worked independently to assess eligibility for inclusion. When necessary, the full text was obtained to determine eligibility. These four reviewers independently conducted the selection of potentially relevant studies by scanning the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches. Ineligible reports were discarded, and the full article was obtained for all potentially relevant reports. The four reviewers independently applied the inclusion criteria with a fifth reviewer acting as arbiter where there was disagreement. Studies were reviewed for eligibility, based on study design, types of participants, coverage and outcomes measures. For studies in which additional information was needed for inclusion in the review, attempts to contact study authors for further clarification of data were made. Sixty-two full-text articles were closely examined by four reviewers and discussed at length among all reviewers through regular conference call meetings. Interventions addressing HCV infection were identified and presented in conjunction with HIV data; additionally, measurements documenting biomarker changes in co-infection with HIV and HCV over time are also presented in this review, if reported by study authors.
Results
Selection of Studies
Searches were conducted on March 15, 2011, and produced 1,831 references; 347 duplicates were removed (Fig. 2). After initial exclusion of 193 titles, 1,291 titles and abstracts were selected for further review by four reviewers.
Results of the Search
After removing duplicates and ineligible citations, 15 studies met the inclusion criteria based on documentation of sufficient NSP coverage of the injecting population, and reliable biomarker information during the period of NSP implementation or scaling-up in a respective location. These 15 studies described ten distinct NSP interventions. If multiple locations in the same state or country were examined, the data were presented by location and by year where appropriate. Included studies cover locations in Australia, Canada, China, France, Ireland, Spain, United Kingdom, United States and Vietnam.
Included Studies
Among the studies included in this review, five studies were conducted in Europe [25–29], two in South East Asia [30, 31], five in the United States [32–36], two in Canada [37, 38] and one in Australia [39]. As there were a small number of studies eligible for inclusion in this review, we chose to describe each study in a narrative review format.
Risk of Bias in Included Studies
All studies were non-randomized before-after comparisons or interrupted time series surveys. The populations addressed in all studies were recruited from their respective communities using different sampling strategies including convenience and systematic sampling of PWID in the respective NSP locations. Six studies showed evidence of participation bias [25, 26, 28, 30, 31, 34], while six studies showed evidence of recruitment bias [25, 26, 28, 32–34]. Three studies did not sufficiently describe follow up results [29, 34, 39].
Description of Studies
The following studies were included in this review: Goldberg et al. [25, 26], Smyth et al. [27], Des Jarlais et al. [32–36], Hope et al. [28], Hammett et al. [30], Des Jarlais et al. [31], Ramirez-Jonville [29], Kerr et al. [37], Bruneau et al. [38] and Topp et al. [39].
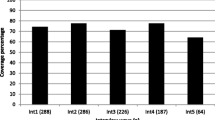
Below we describe the ten distinct interventions collected in our literature search. Several studies described the same intervention; therefore, while we included 15 different studies in this review, the studies come from ten distinct NSP interventions. In locations where multiple studies describe the same intervention, we chose to describe the most complete intervention study with the longest period of follow-up and the most comprehensive review of biomarker information including effect modifiers, adjustment for confounders, and other important quantitative measures. Table 1 describes each intervention, coverage levels for each NSP location, and changes in HIV or HCV prevalence/incidence. In cases where a study reported biomarker changes for separate locations, we recorded these separately in the table; additionally, we separated biomarker information from each of the ten interventions in Table 1, recording results for HIV prevalence, HIV incidence, HCV Prevalence and HCV incidence separately. A total of 26 different biomarker outcomes were documented from these ten distinct interventions reported in fifteen studies.
Goldberg (Glasgow, Scotland) [25, 26]
Goldberg et al. [25, 26] were coded in conjunction with each other as both studies were by the same author, but collected data for different time periods. The 2001 article extended the original 1998 study with two additional data points measured in 1996 and 1997. The intervention, conducted in Scotland, involved the implementation and scale-up of NSP in Glasgow, established in 1988 as a result of the United Kingdom’s National Health Service (NHS) approving the establishment of NSP. These programs were significantly scaled-up between 1988 and 1992. In Glasgow, the intervention was successful in distributing syringes, starting with 2,600 in 1988, increasing to over 300,000 by 1997.
In Goldberg et al. [25], PWID were separated into groups, one group that had received testing for HCV prior to full implementation of NSP (1988) and the other group had HCV testing after full implementation of NSP. Of the 295 PWID who were tested in 1990, the prevalence of HCV infection was 90 %, while in 1995, among the 370 PWID tested, 77 % were positive for HCV infection (p < 0.001). Goldberg et al. [26] extended Goldberg et al. [25] to include prevalence measures from 1996 to 1997. Among the 312 PWID tested in 1996, HCV infection prevalence had increased slightly from 1995 to 80 %, but in 1997, among the 317 samples tested, HCV infection prevalence had decreased to 68 %, for a net reduction in HCV infection prevalence of 12 % between 1996 and 1997 (p < 0.001) and a reduction of 22 % between 1990 and 1997 (p < 0.001). PWID less than 25 years of age had a more significant reduction in HCV infection prevalence during the study period (48 % reduction, p < 0.001) compared to those that were older than or equal to 25 years of age (9 % reduction, p = 0.06).
Smyth (Dublin, Ireland) [27]
Smyth et al. [27] examined PWID in Dublin, Ireland who began their injecting careers before and after large-scale NSP, to estimate the effect NSP services had on HCV infection prevalence among PWID in the city. PWID were recruited from Trinity Court, an organization that specifically addresses the needs of drug users in the Dublin area and provides HIV and HCV testing and counseling, methadone maintenance treatment, drug detoxification services, needle and syringe distribution, and health care related to infections associated with injection and substance use. Since 1992, any PWID who presented at Trinity Court for treatment has been tested for HCV infection; the data presented in the Smyth article incorporates all PWID who were tested at Trinity court between July 1, 1993 and December 31, 1996. The PWID in the sample were typically older (52.4 % greater than 21 years of age), unemployed (90 %) and male (68.3 %). Approximately 77.6 % identified heroin as their injection drug of choice.
PWID in the study were divided into two groups. The first group was comprised of 172 PWID who began their injecting career prior to January 1, 1994, prior to full NSP establishment. The second group comprised 181 PWID who began injecting after January 1, 1994 when NSP had been fully scaled-up and established in Dublin. Among PWID who began injecting prior to NSP, the prevalence of HCV infection was 64.5 %. PWID who began their injection careers after January 1, 1994 had a HCV infection prevalence of 40.3 %, indicating a 24.2 % decrease in overall HCV infection prevalence between pre and post-NSPPWID (p < 0.001). The adjusted odds ratio for HCV infection among the post-NSPPWID compared to pre-NSPPWID was 0.43 after controlling for demographic and other behavioral risk factors including length of injecting career and type of drug injected (p < 0.001).
Des Jarlais (New York City, USA) [32–36]
NSPs were implemented and expanded significantly in New York City during the early 1990s, with syringe distribution increasing from less than 250,000 in 1990 to over 3,000,000 annually by 2001. Coverage for PWID, including secondary exchange, was above 50 %. Five separate but coordinated studies were conducted at the Beth Israel Detoxification Clinics in New York City, to assess changes in HIV incidence, HIV prevalence, HCV infection prevalence, and HCV/HIV co-infection prevalence among PWID entering treatment during the era of NSP implementation [32–36]. PWID were enrolled in the studies if they entered the detoxification units at Beth Israel Medical Center and agreed to serologic testing for HIV or HCV infection. Studies conducted examined prevalence and incidence prior to and after full NSP implementation and expansion in New York City.
Although there were five studies that evaluated the NSP program, we report on the two most complete analyses of changes in HCV infection or HIV among PWID in New York City.
Des Jarlais et al. [32] examined HIV incidence and prevalence among PWID entering the Beth Israel Detoxification unit between 1990 and 2002. A serial cross sectional study design was used to interview and test PWID in 1990–1992 (n = 791), with subsequent tests occurring in 1993–1995 (n = 686), 1996–1998 (n = 705), and 1999–2002 (n = 1,469). A total of 3651 PWID were included in the study. HIV prevalence at baseline in the PWID sample was 50 %, decreasing to 17 % in 2002 (p < 0.001), for a 33 % overall HIV prevalence decrease. HIV incidence also decreased during the same time period, from 3.55/100 person years (PY) at baseline to 0.77/100 PY in 2002 (p < 0.001).
Des Jarlais et al. [33 ] examined HCV infection among PWID entering Beth Israel Detoxification Clinics between 1990–1991 and 2000–2001 in order to document changes in HCV infection prevalence among PWID who entered detoxification during NSP implementation and those that entered detoxification after NSP had been fully implemented in New York City. A total of 484 PWID were recruited into the study; 72 PWID were part of the baseline 1990–1992 sample, and 412 PWID were part of the follow-up sample in 2000–2001.
Among the 72 PWID recruited into the study that had entered detoxification between 1990 and 1991, HCV infection prevalence was 91 %, and among those PWID who entered detoxification between 2000 and 2001, when NSP was fully implemented, HCV infection prevalence was 62 %, indicating a 29 % decrease (p = 0.034). Among PWID identified as HIV negative, HCV infection prevalence decreased from 80 to 59 % over the study period (p < 0.034); those PWID that were HIV positive documented a decrease in HCV infection prevalence from 100 to 82 % (p < 0.0016). Co-infection with HIV and HCV was also measured as part of the study, and among all PWID, weighted prevalence of HIV/HCV co-infection decreased from 53 to 13 % (p < 0.01).
Hope (England and Wales, United Kingdom) [28]
In 1986, England and Wales began to put NSP in place, in response to the elevated levels of HIV infections in Scotland; services were significantly expanded between 1987 and 1997. The intervention in England and Wales included policy changes in 1986 that led to increased funding coupled with pilot NSP locations in 1987. The number of syringes distributed to PWID reached over 25 million in 1997. Coverage of syringes for PWID included 12 syringes for every PWID per month in England and Wales.
Hope et al. [28] examined changes in the HIV prevalence in the community of England and Wales, during NSP expansion and after full NSP implementation. PWID were recruited from multiple locations throughout England and Wales, beginning with recruitment in London from community settings and harm reduction centers between 1990 and 1993, and again in 1996. In 1997–1998, seven other cities in England were included, and by 2001–2002 recruitment had expanded to include the city of Brighton as well. Locally based fieldworkers from health departments and non-government organizations recruited most of the PWID in the study, and recruitment sites included street locations, social venues, participant homes and NSP. A total of 27,932 PWID were recruited and included in the study; over 25 % of the PWID were from London.
Over the course of data collection, HIV prevalence in England and Wales decreased from 5.92 % in 1990 to 1.37 % in 2003 (p < 0.001). Significant declines in HIV prevalence were documented between 1990 and 1996 in the study; HIV prevalence during this period decreased from 5.9 to 0.6 % (p < 0.001). When PWID were stratified by length of injecting career during this same time period, all samples documented decreases in HIV prevalence. Among those injecting 3 years or less, HIV prevalence decreased from 5 to 0.2 %; PWID who had been injecting between 3 and 5 years documented a decrease in HIV prevalence from 3.1 to 0.4 %; PWID who had been injecting between 6 and 12 years documented a decrease in HIV prevalence from 7.4 to 0.2 %; PWID who had been injecting for more than 12 years documented a decrease in HIV prevalence from 9.5 to 1.5 %. All decreases in HIV prevalence by years of injecting were statistically significant (p < 0.05).
There were no statistically different changes in HIV prevalence when stratified by sex (p = 0.321), but there was a correlation between HIV infection and length of injecting career. HIV prevalence increased from 0.68 % among those with injecting careers of 0–2 years to 3.22 % among those injecting for greater than 15 years (p < 0.001). When stratified by recruitment location, those recruited at harm reduction centers registered lower HIV prevalence (1.02 %) compared to those recruited from the community (5.9 %) (p < 0.001) [28].
Hammett and Des Jarlais (Ning Ming, China and Lang Son, Vietnam) [30, 31]
This intervention took place in the cross-border region of Lang Son Province, Vietnam and Ning Ming County, located in the Guangxi province in China. The intervention in these locations involved packaged harm reduction services, including a pharmacy based voucher program for acquiring clean syringes along with clean injecting equipment and condoms. On average, 10,000 to 15,000 syringes were distributed to PWID per month in each location, serving a population of approximately 3,000 PWID. Two studies reported on changes in HIV prevalence over time in these locations [30, 31]; this review includes the longer, more complete analysis from Des Jarlais et al. [31].
Des Jarlais et al. [31] involved a series of cross-sectional measurements of HIV prevalence and incidence among PWID with measurements taken at baseline, 6, 12, 18, 24, and 36 months after implementation of packaged harm reduction services. Thousand three hundred and seventy-nine current and 457 new PWID (defined as those that had first injected within 3 years of their current age) were included in the China sample; 1,102 current and 416 new PWID were included in the Vietnam sample. Among the PWID in Ning Ming China, HIV prevalence decreased from 17 % at baseline to 14 % after 36 months, for a total HIV reduction of 3 % (p < NS). Among Lang Son Vietnam PWID, HIV prevalence decreased from 41 % at baseline to 27 % after 36 months, for a total HIV reduction of 14 % (p < 0.001).
When examining only new PWID, the HIV prevalence in Ning Ming decreased from 16 % at baseline to 0 % after 36 months and HIV incidence decreased from 11/100 PY at baseline to 0/100 PY after 36 months (p < 0.0093). Among new PWID in Lang Son, the HIV prevalence decreased from 31 % at baseline to 5 % after 36 months and HIV incidence decreased from 20/100PY at baseline to 4/100PY after 36 months (p < 0.0002).
Ramirez-Jonville (France) [29]
NSP programs in France began in the early 1990s, preceded by pharmacy distribution throughout the country. The number of syringes distributed through NSP and pharmacies during the 1990s increased from 14.7 million in 1996 to 17.7 million in 1999. At the same time of increased syringe distribution, buprenorphine was also being implemented as a treatment option for PWID. Ramirez-Jonville [29] examined PWID recruited from multiple settings between 1993 and 2002, including street locations where drug users congregate, drug treatment centers and other drug clinics. This period of analysis coincides with the expansion and significant increases in needle/syringe distribution that began in the early 1990s and extended through to 1999, when large-scale NSP occurred. Among the PWID in France, HIV prevalence decreased from 23 % in 1994 to 14 % in 2002, for a 9 % decrease over the study period. Examining HCV infection prevalence over time, there was an increase from 51 % in 1993 to 73 % in 2002, for a total increase of 22 % over the study period.
Ramirez-Jonville (Spain) [29]
The intervention in Spain began in 1990, with significant NSP expansion between 1996 and 2002; during this time period the NSP locations increased from 401 to over 1,400. At the same time, syringe distribution increased from 1.9 million in 1996 to over 6.2 million by 2002. Along with expanded NSP services, methadone maintenance was also expanded in Spain. Ramirez-Jonville [29] examined PWID recruited from multiple settings between 1993 and 2002, including street locations where drug users congregate, drug treatment centers and other drug clinics. Among the PWID in Spain, HIV prevalence decreased from 38 % in 1996 to 33 % in 2002, for a 5 % decrease over the study period. Examining HCV infection prevalence over time, there was an increase from 65 % in 1993 to 89 % in 2002, for a total increase of 24 % over the study period.
Kerr (Vancouver, Canada) [37]
The intervention in Vancouver, Canada was preceded by changes in policy that allowed for PWID to acquire an unlimited number of syringes from NSP, instead of having to exchange syringes on a one for one basis; this policy change occurred between 1998 and 2003 in Vancouver. According to the NSP coordinators in Vancouver, nearly 89 % of PWID had visited the NSP at least once by 2010, with 0.3 % secondary exchange during the same year. In downtown Vancouver (where NSP sites are located), there were approximately 1,246 PWID and were 1.8 million syringes distributed from NSP locations in 2010 [40].
Kerr et al. [37] examined PWID recruited between 1998 and 2003 through street based and peer based recruitment strategies; a total of 1,229 PWID were surveyed during the study period. Multivariate general estimation (GEE) found that PWID who were part of the sample after 2001 were independently associated with reduction in overall HIV incidence [Adjusted hazard ratio (AHR) = 0.13, 95 % confidence interval: 0.06, 0.31].
Bruneau (Montréal, Canada) [38]
Montreal implemented NSP in 1989, with supplemental distribution of syringes occurring in pharmacy outlets in the early 1990s. By 2005 there were 11 operating NSP in the city serving a population of approximately 12,000 PWID. In 1999, there were 340,000 syringes distributed in the city; by 2007, the city was distributing nearly 800,000 syringes annually. Originally the NSP focused on a distribution limit of 15 syringes per day with a 1 for 1 exchange rate; however, in 1996, this was changed to allow for unlimited distribution and exchanging of syringes at the NSP.
Bruneau et al. [38] examined HIV incidence among PWID who were part of the St. Luc Open Cohort study in Montreal, Canada that started in 1988. The open cohort recruited PWID through street recruitment, chain referral, and recruitment at community centers from 1992 to 2001 and from 2004 to 2008; funding did not allow for recruitment to take place between 2002 and 2003 but HIV incidence measurements were still taken for those that had remained in the cohort from previous recruitment years. PWID in the open cohort were predominately male (80.6 %) and primarily injected cocaine (64.8 %) or heroin (30.6 %). Nearly 62 % reported obtaining 100 % of their syringes from NSP or other sterile syringe sources, such as pharmacies.
Over the course of the study period, the incidence of HIV among PWID decreased from 3.5 infections per 100 PY in 1992 to 1.8 infections per 100 PY in 2008, for a reduction of 1.7 new HIV infections per 100 PY over the course of the study period. After controlling for confounders including sexual behaviors and prostitution, females were 0.52 times more likely to become infected with HIV than males (95 % CI: 0.29–0.95).
Topp (Australia) [39]
The intervention in Australia was implemented in 1986 with the Australian federal government authorizing the establishment of NSP. By 2008 there were 52 NSP available throughout Australia, serving a population of approximately 300,000 PWID, and distributing on average 213 clean syringes per injector per year. Topp et al. [39] examined changes in HIV prevalence among PWID visiting NSP throughout Australia during the period of expanding NSP centers during the early 1990s. PWID were recruited between 1995 and 2009 and were eligible for inclusion if they visited the NSP during the month of October when the survey took place every year. A total of 22,478 PWID were included in the study and had sufficient blood samples for HIV testing. PWID recruited into the study were predominantly male (66 %), heterosexual (85 %), and of younger age (median age: 30 years old). The average injecting career of PWID was 10 years. The prevalence of HIV during this period of increasing NSP access decreased from 1.7 % in 1995 to 1.1 % in 2009, for a total decrease of 0.6 % (p = 0.025). HIV positive PWID were more likely to be male (85 %), above 30 years of age (74 %) and less likely to inject daily (60 %). Additionally, compared to PWID recruited in 1995, PWID recruited between 2007 and 2009 were 0.58 times a likely to be HIV infected (p < 0.05).
Discussion
Using a comprehensive search strategy that included both published and unpublished studies, the review focused on structural-level intervention studies that assessed population-level changes in HIV or HCV infection prevalence and incidence among PWID. The studies included interventions that evaluated changes in policies, laws and regulations in relation to access and availability of sterile injecting equipment, use of public funds for establishing structural level large-scale syringe access programs (NSP), and distribution of sterile equipment at the population level, with a “coverage” of more than 10 or more syringes distributed per PWID per year to at least 50 % of the injecting population in the larger community.
The findings highlight the fact that significant public health benefits can be obtained even when at least 50 % of the injecting population in a community receive at least 10 or more sterile syringes per year. The findings also indicate the importance of establishing structural-level large-scale syringe access programs for HIV prevention, especially early in an epidemic among PWID [41]. It is important to acknowledge that as PWIDs in most countries tend to be neglected, there is a paucity of research focusing on impact of structural interventions on HIV and HCV prevalence and incidence.
Quality of the Evidence
Our review identified a moderate number of intervention studies that focused on population-level outcomes of HIV or HCV infection. The reasons for this limited number stems from the lack of structural-level studies, our definition of the concept, and their effect on population-level changes in HIV or HCV infection incidence/prevalence. The studies included in this review can be categorized into two groups based on study designs: before/after comparisons designs [25–27, 32, 34–37], and time-series cross-sectional designs [28, 30, 31, 33, 38, 39]. Although there are other study designs utilized in NSP reports including case–control studies, prospective or retrospective cohort studies and randomized controlled trials, none of the studies included in this review utilized these types of designs.
There are multiple measures of “coverage” of needle/syringe programs, including the percentage of PWID in the local population who utilize the programs (including both direct participation and indirect participation through “secondary exchange,” the numbers of syringes exchanged per PWID per year in the local population), and the number of syringes exchanged per year compared to the estimated number of injections per year in the local population. However, our review does not permit determining which of these is the “best” measure of coverage, but does indicate that any coverage measure should include the number of syringes distributed and the number of PWID in the local population.
Implications for Further Research
While the results reported here support the emphasis of UNAIDS on national-level comprehensive HIV prevention, care and treatment [42], there is clearly a need for further evidence from low- and middle-income countries, especially those with high levels of HIV/HCV infection among PWID [43]. New research studies conducted in low- and middle-income countries need to include data on structural-level NSP coverage and assess biological outcomes at the population level. Additional reductions in residual prevalence or incidence will depend on expansion of structural level NSP, other interventions, or a combination of interventions.
There is also a need to assess population-level changes in association with programs exclusively focused on pharmacy sale of sterile syringes; only studies with pharmacy sales in conjunction with NSP were included in this review. As antiretroviral therapy for HIV can extend lives of HIV-infected PWID and potentially reduce transmission of HIV, implications of ART effects on population-level changes in HIV should address access to and adherence of PWID to ART. There are new antiretroviral medications for HCV infection that have shown promising sustained viral response (SVR) rates of nearly 90 % [44]. Studies also need to address access to, adherence to, and implications of using medications for HCV infection on population-level changes in HCV infection. Because the effect depends on availability and adoption of treatment for HIV and HCV infection, future studies should assess outcomes associated with behavioral, biomedical, and structural interventions.
It will also be important for future research studies to report the numbers of syringes distributed per PWID in the area. As the effectiveness of structural-level needle/syringe programs is likely to be a direct function of needles and syringes made available to PWID, this information is critical. Many of the research reports we examined did not include this information. Future research should also look into effects of pharmacy access to sterile-syringes on HIV/HCV prevalence and incidence. In some low and middle-income countries sterile syringes may be bought from the pharmacy without prescription. Studies may need to be conducted to assess the effects of pharmacy sales in low- and middle-income countries.
Limitations and Implications for Policy
There were several limitations to this review that are worth noting. Our definition of structural-level interventions excluded those that distributed less than 10 needles/syringes/ PWID/year or did not provide data on the numbers of syringes distributed per PWID. In practice, however, coverage levels in many locations have not only reached, but far exceeded the eligibility criteria specified in this review [45]. As PWID in many locations may remain hidden, it can sometimes be difficult to measure the number in a particular location, thus making syringe coverage estimates difficult, even if the number of syringes distributed is known for a particular area.
We were not able to document any primary studies with an RCT design. Although RCTs are seen as the “gold standard” for research, it would be unethical to conduct this type of trial among NSP participants, given the positive results already documented with respect to NSP programs throughout the world [4]. We were also not able to locate any studies that utilized a retrospective cohort or case–control study designs.
Due to the limited number of studies in this review coupled with the wide variation of outcome measures reported, we chose a narrative review of the structural-level NSP. We did, however, report quantitative data for each study, and noted statistically significant changes in biomarkers over time when reported by the author.
Finally, as the effect sizes reported were conducted over a long period of time, there may have been confounding variables not captured in this review, such as implementation of opioid substitution therapy (OST) or anti-retroviral therapy (ART). The effect of structural-level NSP extends to both those who use the services as well as those who receive sterile injection equipment from their peers who visit the structural-level NSP, referred to as “secondary exchange.” Reducing the risk behaviors of PWID who visit structural level NSP can have a partial “herd immunity” effect that can protect the local PWID population as a whole. Thus, structural-level NSP can be considered population-level interventions and their effect can be best measured by study designs that allow for assessing changes in HIV and HCV infections at the population level. Although there are multiple study designs available for conducting analysis of NSP programs, the designs used in the studies covered by this review (non-randomized before-after comparisons or interrupted time series surveys) allowed for assessments of the NSP programs evaluated.
The studies included in this review are heterogeneous in terms of location, structural-level NSP components, level of implementation, level/extent of coverage, the presence of other HIV prevention programs in the community, and study design. The structural-level NSP employ different implementation strategies and operational procedures. This diversity adds strength because all the studies show population-level changes in HIV and HCV prevalence or incidence, and this experience can be important for locations that are currently experiencing HIV or HCV infection epidemics among their PWID populations. The results of this review indicate that large-scale NSP as a structural-level intervention can reduce population-level HIV and HCV infections. Policy makers can consider the potential benefits of implementing structural-level NSP in locations with high HIV and HCV infection prevalence as an important part of a comprehensive strategy to prevent and control HIV and HCV infections.
Notes
For locations in which study authors specified the number of PWID covered by the exchange, we used that percentage if greater than 50 % as rationale for a structural-level intervention (they also had to provide the number of syringes given). In cases that there was no coverage percentage given, we estimated coverage by taking the number of syringes distributed in the location (estimated or given outright by the author) divided by the number of PWID, and if that result was 10 or more syringes, we considered this to be a structural-level intervention. Finally, in some of the studies, authors not only gave percent coverage, but additionally provided syringe numbers and the number of PWID in the particular location. In these instances, we were able to not only document an estimate number of syringes per PWID, but also give an explicit value for coverage. If we could not establish coverage through the author directly or through supplementary searching, the study was not included in the review as we could not determine the program as structural or not.
Our coverage criteria were based on Vickerman et al. [21], which indicated threshold coverage of more than 20 % to affect substantial decreases in HIV prevalence.
References
Gerberding JL. Incidence and prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and cytomegalovirus among health care personnel at risk for blood exposure: final report from a longitudinal study. J Infect Dis. 1994;170(6):1410–7.
Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP, 2007 Reference Group to the UN on HIV and Injecting Drug Use. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45.
Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–109.
Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105(5):844–59.
Anderson E, Gans J, Shwayder P, Scofield J, Catizone C. HIV prevention and access to sterile syringes. National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention. Atlanta; 1999.
Centers for Disease Control. Report on pharmacy sales of sterile syringes. Atlanta; 2005.
Sheridan J, Henderson C, Greenhill N, Smith A. Pharmacy-based needle exchange in New Zealand: a review of services. Harm Reduct J. 2005;2:10.
Wright-Deaguero L, Weinstein B, Jones TS, Mills J. Impact of the change in Connecticut syringe prescription laws on pharmacy sales and pharmacy managers’ practices. JAIDS. 1998;18(Supp l):S102–10.
Valleroy LA, Weinstein B, Jones TS, Groseclose SL, Rolfs RT, Kassler WJ. Impact of increased legal access to needles and syringes on community pharmacies needle and syringe sales—Connecticut, 1992–1993. JAIDS. 1995;10:73–81.
Somaini B, Wang J, Perozo M, Kuhn F, Meili D, Grob P, et al. A continuing concern: HIV and hepatitis testing and prevalence among drug users in substitution programmes in Zurich, Switzerland. AIDS Care. 2000;12(4):449–60.
Vlahov D, Robertson AM, Strathdee SA. Prevention of HIV infection among injection drug users in resource-limited settings. Clin Infect Dis. 2010;50(Suppl 3):S114–21.
Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS. 2000;14(Suppl 1):S3–10.
Blankenship KM, Friedman SR, Dworkin S, Mantell JE. Structural interventions: concepts, challenges and opportunities for research. J Urban Health. 2006;83(1):59–72.
Rhodes T, Lilly R, Lalam N, Giorgino E, Kemmesis UE, Ossebaard HC, et al. Risk factors associated with drug use: the importance of risk environment. Drugs Educ Prev Policy. 2003;10(4):303–29.
Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–44.
Jones L, Pickering L, Sumnall H, McVeigh J, Bellis MA. Optimal provision of needle and syringe programmes for injecting drug users: a systematic review. Int J Drug Policy. 2010;21(5):335–42.
Wodak A, Cooney A. Effectiveness of sterile needle and syringe programming in reducing HIV/AIDS among injecting drug users. Evidence for Action Technical Papers. Geneva: WHO; 2004.
Garu LE, Bluthenthal RN, Marshall P, Singer M, Heimer R. Psychosocial and behavioral differences among drug injectors who use and do not use syringe exchange programs. AIDS Behav. 2005;9(4):495–504.
Sharma M, Burrows D, Bluthenthal R. Coverage of HIV prevention programmes for injection drug users: confusions, aspirations, definitions and ways forward. Int J Drug Policy. 2007;18:92–8.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 5th ed. West Sussex: The Cochrane Collaboration and Wiley; 2008.
Vickerman P, Hickman M, Rhodes T, Watts C. Model projections on the required coverage of syringe distribution to prevent HIV epidemics among injecting drug users. J Acquir Immune Defic Syndr. 2006;42(3):355–61.
AIDS Information and Global Education System. AIDS information and global education system from http://www.aegis.com/ 2011.
Denison JA, O’Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS Behav. 2008;12(3):363–73.
Kennedy CE, Spaulding AB, Brickley DB, Almers L, Mirjahangir J, Packel L, et al. Linking sexual and reproductive health and HIV interventions: a systematic review. J Int AIDS Soc. 2010;13:26.
Goldberg D, Cameron S, McMenamin J. Hepatitis C virus antibody prevalence among injecting drug users in Glasgow has fallen but remains high. Commun Dis Public Health. 1998;1(2):95–7.
Goldberg D, Burns S, Taylor A, Cameron S, Hargreaves D, Hutchinson S. Trends in HCV prevalence among injecting drug users in Glasgow and Edinburgh during the era of needle/syringe exchange. Scand J Infect Dis. 2001;33(6):457–61.
Smyth BP, Keenan E, O’Connor JJ. Evaluation of the impact of Dublin’s expanded harm reduction programme on prevalence of hepatitis C among short-term injecting drug users. J Epidemiol Community Health. 1999;53(7):434–5.
Hope VD, Judd A, Hickman M, Sutton A, Stimson GV, Parry JV, et al. HIV prevalence among injecting drug users in England and Wales 1990 to 2003: evidence for increased transmission in recent years. AIDS. 2005;19(11):1207–14.
Ramirez-Jonville A. Drug addiction: harm reduction policies in France and Spain. Presse Med. 2006;35(7–8):1151–61.
Hammett TM, Kling R, Johnston P, Liu W, Ngu D, Friedmann P, et al. Patterns of HIV prevalence and HIV risk behaviors among injection drug users prior to and 24 months following implementation of cross-border HIV prevention interventions in northern Vietnam and southern China. AIDS Educ Prev. 2006;18(2):97–115.
Des Jarlais DC, Kling R, Hammett TM, Ngu D, Liu W, Chen Y, et al. Reducing HIV infection among new injecting drug users in the China-Vietnam Cross Border Project. AIDS. 2007;21(Suppl 8):S109–14.
Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95(8):1439–44.
Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Hagan H, Beatrice S, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19(Suppl 3):S20–5.
Des Jarlais DC, Arasteh K, Hagan H, McKnight C, Perlman DC, Friedman SR. Persistence and change in disparities in HIV infection among injection drug users in New York City after large-scale syringe exchange programs. Am J Public Health. 2009;99(Suppl 2):S445–51.
Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101(1–2):88–91.
Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman DC, Torian LV, et al. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug Alcohol Depend. 2010;109(1–3):154–60.
Kerr T, Small W, Buchner C, Zhang R, Li K, Montaner J, et al. Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health. 2010;100(8):1449–53.
Bruneau J, Daniel M, Abrahamowicz M, Zang G, Lamothe F, Vincelette J. Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in Montreal, Canada: a 16-year longitudinal study. Am J Epidemiol. 2011;173(9):1049–58.
Topp L, Day CA, Iversen J, Wand H, Maher L. Fifteen years of HIV surveillance among people who inject drugs: the Australian Needle and Syringe Program Survey 1995–2009. AIDS. 2011;25(6):835–42.
Personal communication with Kerr on May 21 2010.
Lurie P, Drucker E. An opportunity lost: HIV infections associated with lack of a national needle-exchange programme in the USA. Lancet. 1997;349(9052):604–8.
UNAIDS. Practical guidelines for intensifying HIV prevention: towards universal access. Geneva: UNAIDS; 2007.
GMHC. Syringe exchange programs around the world: the global context. P. Sean Cahill, and Nathan Schaefer, Gay Men’s Health Crisis. http://www.gmhc.org/files/editor/file/gmhc_intl_seps.pdf 2009.
Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–28.
Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–28.
Acknowledgments
The project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) as part of country activities. Time and effort of Dr. Des Jarlais, Jonathan Feelemyer and Shilpa Modi were supported through US NIH Grant R01 AI 083035.
Conflict of interest
Dr. Don Des Jarlais was an investigator on six of the included studies Des Jarlais et al. [31–36]. Dr. Des Jarlais affirms that he has no financial or any other tangible interest in his studies being reviewed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdul-Quader, A.S., Feelemyer, J., Modi, S. et al. Effectiveness of Structural-Level Needle/Syringe Programs to Reduce HCV and HIV Infection Among People Who Inject Drugs: A Systematic Review. AIDS Behav 17, 2878–2892 (2013). https://doi.org/10.1007/s10461-013-0593-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-013-0593-y