Abstract
The purpose of this analysis was to estimate human immunodeficiency virus (HIV) prevalence and testing patterns among injection drug users (IDUs) in St. Petersburg, Russia. HIV prevalence among 387 IDUs in the sample was 50%. Correlates of HIV-positive serostatus included unemployment, recent unsafe injections, and history/current sexually transmitted infection. Seventy-six percent had been HIV tested, but only 22% of those who did not report HIV-positive serostatus had been tested in the past 12 months and received their test result. Correlates of this measure included recent doctor visit and having been in prison or jail among men. Among the 193 HIV-infected participants, 36% were aware of their HIV-positive serostatus. HIV prevalence is high and continuing to increase in this population. Adequate coverage of HIV testing has not been achieved, resulting in poor knowledge of positive serostatus. Efforts are needed to better understand motivating and deterring factors for HIV testing in this setting.
Similar content being viewed by others
Introduction
Testing for human immunodeficiency virus (HIV) infection to increase individuals’ awareness of HIV-positive serostatus is a key component of the global strategy to reduce the impact of the AIDS pandemic (Janssen et al. 2001; UNAIDS 2004; WHO 2002). Early detection of HIV infection in individuals is essential for providing linkages to HIV-related support, care, and treatment, and for preventing further spread of infection. Individuals who know they are infected can adopt behaviors such as reducing the frequency of unprotected intercourse and not sharing injecting equipment that will reduce the likelihood of transmission to other individuals. A recent meta-analysis of studies in the United States found that individuals substantially reduce HIV sex risk behaviors after becoming aware of HIV-positive serostatus (Marks et al. 2005). Furthermore, another meta-analysis concluded that behavioral interventions are efficacious in reducing unprotected sex acts among HIV-positive individuals (Crepaz et al. 2006). Another way in which knowledge of HIV-positive serostatus may reduce HIV risk behaviors is through disclosure of positive serostatus to sex or drug partners so that partners may make informed decisions about risk-reduction strategies. Though the evidence for risk reduction in partnerships in which disclosure has occurred is more equivocal (Crepaz and Marks 2002), at a minimum it allows for a discussion about risk reduction. Finally, there is strong evidence that injection drug users (IDUs) adopt drug-related HIV risk-reduction strategies such as lowered rates of distributive needle and syringe sharing when they know they are HIV-positive; this is known as “informed altruism” (Des Jarlais et al. 2004).
The Russian Federation is an important context in which to examine HIV testing and knowledge of HIV-positive serostatus. Russia has had explosive growth in its HIV epidemic since experiencing the dramatic social and economic change that began in the 1990s. According to the Russian Federal AIDS Center, the cumulative number of officially registered people with HIV/AIDS increased from ≈2,000 to 417,715 from 1996 to 2007 (AFEW 2007), making the epidemic in Russia one of the fastest growing epidemics observed anywhere in the world (WHO 2005). An estimated 560,000–1,600,000 individuals are currently living with HIV/AIDS, and the adult prevalence is estimated to be approximately 1% (WHO 2006). The epidemic has been concentrated largely among IDUs (Lowndes et al. 2003; Rhodes et al. 2002; Shaboltas et al. 2006), and currently, IDUs comprise approximately 85% of the cumulative number of registered AIDS cases (WHO 2005). The HIV epidemic in St. Petersburg, one of the largest cities in Russia (population: 4.2 million), mirrors that of the Russian Federation with HIV prevalence among select samples of IDU increasing from <5% prior to 2000 to 30% in 2003 (Abdala et al. 2003; Shaboltas et al. 2006). This dramatic increase in prevalence indicates a very high incidence and is clearly cause for concern.
Little is known about HIV testing patterns and knowledge of HIV-positive serostatus among IDUs in St. Petersburg. Therefore, the objectives of the present analyses were to: (1) provide an updated estimate of HIV prevalence among IDUs for the city of St. Petersburg, (2) describe HIV testing patterns and correlates, and (3) estimate accuracy of knowledge of positive serostatus. These results are needed to better understand the course of the HIV epidemic in St. Petersburg and to develop targeted areas for prevention.
Methods
These analyses were based on data collected as part of a larger and ongoing multi-site study called Sexual Acquisition and Transmission of HIV-Cooperative Agreement Program (SATH-CAP). The study takes place in St. Petersburg, Russia and three other field sites in the USA; present analyses are restricted to data collected at the St. Petersburg site from November 2005–December 2006. The overall goal of SATH-CAP is to better understand how patterns of sexual and drug use behaviors along with other social and environmental factors influence the spread of HIV/AIDS from people currently at high risk for HIV/AIDS (drug users (DUs) and men who have sex with men (MSM)) to other members of the general population.
Participants
The goal of participant recruitment was to achieve a representative sample of DU from the city of St. Petersburg. Participants were recruited into the SATH-CAP study using a dual-component form of respondent driven sampling (RDS) (Heckathorn 1997, 2007). RDS is a chain referral sampling methodology that uses dual incentives and structured coupon disbursement procedures for peer referrals in order to reduce biases that are typically inherent in samples of hidden populations. Traditional RDS methods were modified in this study to recruit both additional members of the target population (DUs and MSM) as well as sex partners of participants. Briefly, a number of “seeds” who were known DUs or MSM were recruited by the study staff, enrolled, and given coupons with study location information to give to their peers (other members of the target population and their sex partners). Initial seeds included participants from previous studies at the research site (Shaboltas et al. 2006) or referrals from agencies providing services to DUs. Peers and sex partners then called the site to schedule an appointment and presented the coupon at the study site for eligibility screening and enrollment. When potential participants arrived at the project site, the purpose of the study and study procedures were explained, and interested individuals provided informed consent. Participation was confidential. Upon completion of all study procedures, participants were reimbursed with gifts worth approximately US$10 containing cell phone cards or personal care items. They were also given subway tokens, condoms and HIV prevention information.
Forty-eight seeds were enrolled, and 35 (73%) recruited at least one other participant. Among those who recruited at least on other participant (“productive seeds”), the mean number of participants recruited from a single seed’s chain of recruits was 5 and the median was 14. The maximum number of people enrolled in a single seed’s chain was 165 (minimum = 1). The distribution of the number of waves, which can be thought of as “steps” from the seed, in this sample was as follows: mean: 2.8, median = 2, minimum = 1, maximum = 14. A total of 522 participants were enrolled. The eligibility criterion for inclusion in the present analysis was reporting ever having injected drugs, which included 387 participants (non-IDUs, MSM, and sex partners were excluded).
Measures
Participants completed structured interviews using computer assisted survey interviewing technology on laptop computers that took approximately 60–120 min to complete. Data used in the present analyses included demographics, drug and sex behaviors, structural and health service utilization, and HIV testing history measures. Demographic variables included sex, age, and current housing and employment status. Drug use behaviors included use in the past 30 days, age at first use, duration of use, frequency of use in past 30 days, number of IDU partners in the past 30 days, having a new IDU partner in past 30 days, and unsafe injections in past 30 days (defined as sharing needles without using bleach, using a single syringe to mix or divide drugs, or sharing cookers, cotton or water). Sex behaviors included age at first sex, number of sex partners in past 6 months, new sex partners in past 6 months, having ever had sex with a male (among men), and having ever exchanged money or goods for sex (among women), and having ever been pregnant. Structural and service utilization variables included history of incarceration, number of doctor visits in past year, having health insurance, and having ever received drug treatment. Questions about HIV testing included having ever been previously tested for HIV, number of times tested, date of last test, and knowledge of test results.
Results from HIV and sexually transmitted infection (STI) testing were also included in the present analyses. Pre-test HIV counseling was conducted, a blood specimen was collected to test for HIV, syphilis, hepatitis B and C viruses, and a urine specimen was collected to test for chlamydia and gonorrhea. Serum specimens were tested for HIV antibodies by enzyme-linked immunosorbent assay (ELISA) using commercially licensed reagents including Genscreen HIV 1/2 (BioRad, France) and/or Vironostika HIV Uni-Form II plus 0 (Biomerieux, The Netherlands). Non-reactive specimens were considered to be HIV-negative. Specimens that were reactive were further tested with Western blot analysis using New Lav Blot HIV-1 (BioRad, France). STI testing was done for syphilis, gonorrhea, and chlamydia. Serum samples were tested for syphilis with rapid plasma reagin test (RPR) (Macro-Vue RPR Card Tests, Becton Dickinson, USA) and Treponema pallidum particle agglutination assay (TPPA) (Serodia-TPPA, Fujirebio, Japan); samples were considered positive for history of syphilis if reactive on both tests. Urine specimens were tested for chlamydia and gonorrhea using polymerase chain reaction (Amplicor CT/NG, Roche, USA). All participants were invited to return to the site for post-test counseling to get HIV and STI test results, and referrals for additional medical and social services were provided as needed.
Statistical Analysis
The sample was described on demographic, drug use and sex behaviors, and service utilization variables using standard descriptive statistics. Participants were considered HIV-positive if their ELISA reactive specimens were confirmed by Western blot. To estimate correlates of HIV-positive serostatus, bivariate and multivariate logistic regression were used. Examined correlates included the demographic, drug use and sex behaviors, prevalent STI variables, and structural and service utilization variables described above. Our initial modeling strategy was intended to be an exploratory analysis of behavioral correlates. The finding that none of the behavioral correlates were associated led us to speculate that structural factors may be important; thus our modeling strategy was revised to include structural variables. Continuous variables were dichotomized for two reasons. First, because the outcome of interest was dichotomous, the logistic regression modeling produces estimates that can be interpreted as odds ratios; these estimates are most easily interpreted with dichotomous covariates that compare one level of the covariate to the other level. Second, dichotomizing covariates provides greater statistical power in the regression models by minimizing the levels of stratification. We used a priori meaningful cut points for dichotomizing. Covariates that were associated in the bivariate analysis using the critical value of P < 0.20 were included in the initial multivariate model. A backward selection procedure eliminated covariates that did not remain significant using the critical value of P < 0.05 to arrive at the final multivariate model.
Patterns of HIV testing were described using proportions and medians to estimate the prevalence of having ever been tested, the frequency of testing, time since last test, and knowledge of positive serostatus. Bivariate and multivariate logistic regression were used to determine correlates of having had a recent HIV test (in the past 12 months) and having received results among individuals who self-reported being HIV-negative using the same modeling strategy described above. To estimate the accuracy of knowledge of positive HIV serostatus, we related self-reported status to laboratory results using blood specimens collected at the time of the interview. We estimated with a 95% confidence interval the proportion of HIV-infected individuals who self-reported correct knowledge of their HIV-positive status.
Because we used RDS for recruitment of IDUs, weighting procedures are available (RDS Analyzing Tool, RDSAT software, version 5.6) to adjust the sample for recruitment probabilities to obtain estimates that are representative of the underlying target population. Using a weighting procedure that took into account our modified RDS methodology, we found that the weighted and unweighted estimates of sex, age, and HIV status proportions in this sample did not differ substantially: unweighted estimates for proportions male, <25 years of age, and HIV-infected were 74%, 33% and 50%, respectively, compared to weighted estimates of 75%, 32%, and 49%, respectively. Furthermore, estimation of 95% confidence intervals for each of these estimates indicated substantial overlap and a similar range between weighted and unweighted bounds due to virtually identical standard deviations of weighted and unweighted estimates (data not shown). Therefore, we used unweighted estimates for all subsequent analyses.
Results
Participant Characteristics and HIV Prevalence
The sample (n = 387) was predominantly male (74%) and the mean age was 28.8 (standard deviation: 6.5) years. Nearly one-third (31%) of the sample was currently employed full- or part-time. The vast majority of the sample, 91%, reported injection drug use in the past 30 days, and 80% reported injecting heroin. Nearly half the sample (48%) reported age at first injection 18 years or younger. The median time since initiating injection drug use was 8 years, and 65% reported at least one instance of some form of unsafe injection in the past 30 days. Age at first sex was 15 years or younger for 53% of the sample, 38% had multiple sex partners, and 49% had a new sex partner in the past 6 months. Eleven male IDUs (4%) reported having ever had male sex partners and 7 female IDUs (7%) reported having ever received money or other goods as payment for sex; due to these low numbers, these factors were not considered covariates in subsequent analyses. Health care utilization characteristics are as follows: 56% had a doctor visit in the past 12 months, 79% had health insurance, and 30% had ever received drug treatment. Thirty-seven percent of the sample had ever been in prison or jail.
Eighteen percent (n = 68) of the sample tested positive for syphilis, chlamydia, or gonorrhea. A total of 196 specimens were ELISA positive for HIV and 1 was indeterminate. Of the 197 specimens tested by Western blot, 193 were positive, 3 were indeterminate, and 1 was negative. We considered the indeterminate results to be negative (by excluding them from the numerator but including them in the denominator) to produce a conservative estimate, and thus estimated HIV prevalence to be 50% (193/387). A total of 290 participants (75%) returned for their HIV test results. Correlates are presented in Table 1. In the adjusted analysis, current unemployment (OR = 1.97, 95% CI = 1.26–3.08, P < .01), reporting unsafe injections in past 30 days (OR = 1.54, 95% CI = 1.0, 2.36, P < .05), and prevalent infection with chlamydia or gonorrhea or prevalent or past diagnosis of syphilis (OR = 1.96, 95% CI = 1.12–3.42, P < .05) were associated with HIV-positive serostatus.
HIV Testing Patterns and Correlates
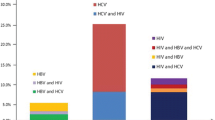
Seventy-six percent (76%) of the sample reported having ever been tested for HIV prior to enrollment in the present study. Of those who had been tested (n = 295), the median number of previous tests was 2 (range: 1–55), and 36% of the sample had only been tested once. The median time since last test was 8 months (range: 0–96), and 30% had not been tested in the past 12 months. Twenty-four percent of those who had been tested reported knowing that they were HIV-positive, 60% reported that they were HIV-negative, and 17% did not know their status (Fig. 1).
To examine factors associated with HIV testing patterns, we considered the outcome of interest to have been tested in the past year and received test results among those who did not self-reported HIV-positive serostatus (n = 315). Sixty-nine individuals (22%) had been tested in the past 12 months and received their test result. Results of the bivariate analysis are presented in Table 2. To account for the association with having ever been pregnant, a multivariate model on the entire sample was run without this covariate, and stratified models were run for men and women separately (Table 3). In the entire sample, only having a doctor visit in the past 12 months remained significantly associated with recent testing (OR = 3.06, 95% CI = 1.68–5.59, P < .01). Among men, having a doctor visit in the past 12 months (OR = 3.66, 95% CI = 1.75–7.70, P < .01) and history of incarceration (OR = 2.12, 95% CI = 1.08–4.42, P < .05) were associated with recent testing, and among women having ever been pregnant was marginally associated (OR = 3.56, 95% CI = 0.74–17.2, P < .10).
Knowledge of HIV-Positive Serostatus
Accuracy of knowledge of positive serostatus is depicted Fig. 1. Of the 72 who self-reported positive serostatus, 70 (97%) were HIV-positive by serology. Among the 315 participants who did not report positive serostatus, 123 (24%) were HIV-positive. This included 30 participants who reported not knowing their status (60% of this group), 52 participants who reported having never been tested (57% of this group), and 41 participants who reported they were HIV-negative (24% of this group). The proportion of HIV-infected participants who self-reported that they knew they were HIV-infected was 36% (70/193) with a 95% confidence interval of 30–43%. In other words, 64% (123/193) of HIV-positive individuals in this sample did not know their HIV status.
Discussion
A key finding of this study is the HIV prevalence estimate of 50% among IDUs in St. Petersburg, Russia. This updated estimate shows steady increase in comparison with previous estimates of 11% from data collected in 2000 (Abdala et al. 2003), and 30% from data collected in 2002 (Shaboltas et al. 2006), and indicates continued growth of this explosive epidemic. It is noteworthy that the study conducted in 2002 also relied heavily on chain referral sampling to access a similar population, suggesting a degree of comparability in these samples. Though we are unable to assess the temporal relationships between covariates and HIV status due to the cross-sectional nature of this study, the significantly higher reporting of recent unsafe injections among those who are HIV-positive signals concern for continued transmission. While we do not know the injection behaviors of participants during the time in which they acquired HIV, their current behaviors suggest that the potential for secondary transmission to their drug partners is high. Similarly, the association between STI (history of syphilis or current chlamydia/gonorrhea) and HIV infection suggests a possible role of sexual transmission of HIV among IDU in this population, either as a risk factor in the past or signaling the potential for future spread. The extent to which the epidemic will continue to grow and spread to the general population is an important and urgent question that the larger study on which this analysis was based is designed to address.
A substantial proportion of IDUs in this sample from St. Petersburg had not been tested for HIV, ever or recently, or received their test results. Overall, 24% had never been tested, and among those who had been tested, 30% had not done so in the past year and 17% had not received test results. These results are comparable to earlier findings from other Russian cities. One study conducted in 2003 in the three Russian cities of Moscow, Barnaul, and Volgograd reported that 25–48% of IDUs had not been tested for HIV in the past year and that 66% of HIV-positive IDUs were unaware of their HIV-positive serostatus (Rhodes et al. 2006). Our findings contribute to this growing body of knowledge by examining testing patterns in a different city and by examining correlates; neither of which had previously been done. Also, given the highly dynamic and rapidly increasing HIV epidemic in Russia, our analysis that provides an updated estimate is needed to continually monitor the situation. Sadly, our findings suggest that previously limited testing coverage has not increased over time despite continued growth of the epidemic. It should also be noted that our estimate of 24% having never been tested is substantially higher than the 7% estimate from 5 US cities (Heimer et al. 2007). This relatively low frequency of HIV testing has persisted in a high-risk group despite an emphasis on routine HIV testing in Russia. According to the WHO, approximately 20 million HIV tests are conducted annually in Russia (WHO 2005), a country that has a total population of approximately 142 million. Though voluntary counseling and testing (VCT) is guaranteed by Russian law, this may not be widely accessible or conducted on a truly voluntary basis. Furthermore, IDUs may not seek HIV testing for a variety of reasons. For example, substantial stigma in the general population toward people with HIV/AIDS may make knowing ones’ status undesirable (Balabanova et al. 2006). Lack of access to affordable antiretroviral treatment may be another barrier to accessing VCT to learn one’s status. The WHO currently estimates that approximately 10% of those in need of antiretroviral treatment receive it in Russia (WHO 2006), in large part because generic drugs are not registered and treatment is not affordable to many Russians. However, increased governmental commitments to and financial support for treatment in Russia have recently occurred, and these efforts are likely to increase the proportion of people who may receive treatment. As this occurs, the acceptability of HIV testing becomes increasingly important.
The finding that none of the demographic or drug and sex risk behaviors was correlated with HIV testing patterns is evidence of our limited understanding of motivations for and barriers to HIV testing. The lack of identified behavioral correlates in the present study may reflect the large volume of routine tests that is conducted in Russia. This is supported by our finding that having a recent doctor visit, history of incarceration, and having been pregnant—all events that provide contact with institutions (medical or other) that provide routine testing—were in fact associated with recent testing. It is possible that structural characteristics are more important determinants of HIV testing than individual preferences though this has not been adequately studied.
Despite the large-scale national investment in routine HIV testing, the low testing and return rates have resulted in poor knowledge of positive HIV serostatus among IDUs in St. Petersburg. We found that 24% of individuals who self-reported that they were HIV-negative were actually HIV-positive; this proportion was substantially higher among individuals who had been tested but not received results (60%) and individuals who had never been tested (57%). Overall, nearly two-thirds (64%) of HIV-infected individuals did not know their positive serostatus. These findings are also comparable to findings in other studies from the region, including the same proportion (two-thirds) of IDUs being unaware of positive HIV status in the Russian three city study (Rhodes et al. 2006) and three-quarters of IDUs being unaware of their positive status in another Russian city (Rhodes et al. 2002). Again, it is discouraging that these estimates have not improved as the epidemic has continued to expand. These findings have implications for secondary prevention in that individuals who do not know they are HIV-positive may not ad opt risk-reduction behaviors that can prevent transmission of HIV to their drug and sex partners. The usefulness of this type of secondary prevention has been evaluated and reviewed in other countries (Crepaz et al. 2006; Marks et al. 2005) but has not been evaluated in the Russian setting.
Several possible limitations of these findings should be noted. First, our results may not generalize to IDUs outside of St. Petersburg, though they may apply to areas of similar epidemiologic characteristics, and others are encouraged to consider the applicability of these findings to their own settings. Given the recent rapid rise in HIV in this region and the possible substantial role this region may play in the current wave of the HIV pandemic, our findings provide insight into strategies that may be needed to reduce the impact of HIV, including HIV testing. Second, though our recruitment strategy (RDS) is designed to achieve a representative sample of the underlying population, the degree to which this occurred is unknown, although the initial weighting analysis suggests heterophily at a level consistent with achieving representativeness (Heckathorn 2002). Third, HIV testing history was obtained by self-report and may be subject to unintentionally inaccurate responses due to misreport or forgetting. On the other hand, the effect of intentional misreport due to social desirability should be minimized by the anonymous nature of data collection and use of CASI technology. Fourth, the lack of identified significant correlates of HIV testing patterns may be due to insufficient sample size for the required statistical power or loss of information resulting from dichotomization of covariates. Despite these potential methodological limitations, these results present the first look at HIV testing patterns among IDU in St. Petersburg, a large city with a high burden of HIV infection, and an updated prevalence estimate from earlier reports.
Conclusions
These data show sub-optimal coverage of HIV testing in a high-risk population in a setting of explosive growth, and they also reflect our limited understanding of motivations and deterrents for HIV testing. Future work must address targeted testing and counseling programs and understanding attitudes toward HIV testing by IDUs in Russia, including perceptions of the relative balance between potential benefits and harms. This will become increasingly important as progress in antiretroviral scale-up increases. Until this occurs, emphasizing the potential benefit of knowing one’s status for secondary prevention may be useful. Finally, addressing the issue of stigma toward people living with HIV/AIDS is another great, but necessary, challenge in increasing the acceptability of HIV testing among IDUs in Russia.
References
Abdala, N., Carney, J. M., Durante, A. J., Klimov, N., Ostrovski, D., Somlai, A. M., et al. (2003). Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St. Petersburg, Russia. International Journal of STD and AIDS, 14, 697–703. doi:10.1258/095646203322387965.
AIDS Foundation East-West. (2007). Officially registered HIV cases in Russian Federation 1 January 1987–31 May 2007 as reported by the Russian Federal AIDS Center. Available at http://www.afew.org. Accessed 29 September 2008
Balabanova, Y., Coker, R., Atun, R. A., & Drobniewski, F. (2006). Stigma and HIV infection in Russia. AIDS Care, 18, 846–852. doi:10.1080/09540120600643641.
Crepaz, N., Lyles, C. M., Wolitski, R. J., Passin, W. F., Rama, S. M., Herbst, J. H., et al. (2006). Do prevention interventions reduce HIV risk behaviors among people living with HIV? A metanalytic review of controlled trials. AIDS (London, England), 20, 143–157. doi:10.1097/01.aids.0000196166.48518.a0.
Crepaz, N., & Marks, G. (2002). Towards an understanding of sexual risk behavior in people living with HIV: A review of social, psychological, and medical findings. AIDS (London, England), 16, 135–149. doi:10.1097/00002030-200201250-00002.
Des Jarlais, D. C., Perlis, T., Arasteh, K., Hagan, H., Milliken, J., Braine, N., et al. (2004). “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. Journal of Acquired Immune Deficiency Syndromes, 35, 158–166. doi:10.1097/00126334-200402010-00010.
Heckathorn, D. D. (1997). Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems, 44, 174–199. doi:10.1525/sp.1997.44.2.03x0221m.
Heckathorn, D. D. (2002). Respondent-driven sampling II: Deriving valid population estimates from chain referral samples of hidden populations. Social Problems, 49, 11–34. doi:10.1525/sp.2002.49.1.11.
Heckathorn, D. D. (2007). Extensions of respondent-driven sampling: Analyzing continuous variables and controlling for differential recruitment. Sociological Methodology, 37, 151–208. doi:10.1111/j.1467-9531.2007.00188.x.
Heimer, R., Grau, L. E., Curtin, E., Khoshnood, K., & Singer, M. (2007). Assessment of HIV testing of urban injection drug users: Implications for expansion of HIV testing and prevention efforts. American Journal of Public Health, 97, 110–116. doi:10.2105/AJPH.2005.078105.
Janssen, R. S., Holtgrave, D. R., & Valdiserri, R. O. (2001). The serostatus approach to fighting the HIV epidemic: prevention strategies for infected individuals. American Journal of Public Health, 91, 1019–1024.
Lowndes, C. M., Renton, A., Alary, M., Rhodes, T., Garnett, G., & Stimson, G. (2003). Conditions for widespread heterosexual spread of HIV in the Russian Federation: Implications for research, monitoring and prevention. The International Journal on Drug Policy, 14, 45–62. doi:10.1016/S0955-3959(02)00208-6.
Marks, G., Crepaz, N., Senterfitt, J. W., & Janssen, R. S. (2005). Meta-analysis of high-risk sexual behaviors in persons aware and unaware they are infected with HIV in the United States. Journal of Acquired Immune Deficiency Syndromes, 39, 446–453. doi:10.1097/01.qai.0000151079.33935.79.
Rhodes, T., Lowndes, C. M., Judd, A., Mikhailova, L. A., Sarang, A., Rylkov, A., et al. (2002). Explosive spread and high prevalence of HIV infection among injecting drug users in Togliatti City, Russia. AIDS (London, England), 16, F25–F31. doi:10.1097/00002030-200209060-00002.
Rhodes, T., Platt, L., Maximova, S., Koshkina, E., Latishevskaya, N., Hickman, M., et al. (2006). Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: A multi-city study. Addiction (Abingdon, England), 101, 252–266. doi:10.1111/j.1360-0443.2006.01317.x.
Shaboltas, A. V., Toussova, O. V., Hoffman, I. F., Heimer, R., Verevochkin, S. V., Ryder, R. W., et al. (2006). HIV prevalence, social demographic and behavioral correlates and recruitment methods among injection drug users in St. Petersburg, Russia. Journal of Acquired Immununodeficiency Syndromes, 41, 657–663.
United Nations Joint Program on HIV/AIDS. (2004). UNAIDS/WHO policy statement on HIV testing.
World Health Organization. (2002). WHO consultation on increasing access to HIV testing and counseling.
World Health Organization. (2005). Russian Federation: Summary country profile for HIV/AIDS treatment scale-up.
World Health Organization. (2006). Epidemiologic fact sheet 2006 Russian Federation.
Acknowledgements
This work was supported by a grant from National Institutes of Health (U01DA017387) to Yale School of Medicine and The Biomedical Center. The authors acknowledge the dedication of the SATH-CAP research team at the Biomedical Center, St. Petersburg, Russia. The authors also thank investigators at other sites of the SATH-CAP research project including RAND Corporation, Research Triangle Institute, University of California at Los Angeles, and University of Illinois Chicago.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niccolai, L.M., Toussova, O.V., Verevochkin, S.V. et al. High HIV Prevalence, Suboptimal HIV Testing, and Low Knowledge of HIV-Positive Serostatus Among Injection Drug Users in St. Petersburg, Russia. AIDS Behav 14, 932–941 (2010). https://doi.org/10.1007/s10461-008-9469-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-008-9469-y