Abstract
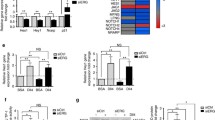
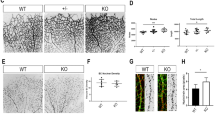
Angiogenesis is a multistep process that requires highly regulated endothelial cell (EC) behavior. The transcription factor Krüppel-like factor 4 (KLF4) is a critical regulator of several basic EC functions; we have recently shown that KLF4 disturbs pathological (tumor) angiogenesis by mediating the expression of members of VEGF and Notch signaling pathways. Notch signaling is central to orchestration of sprouting angiogenesis but little is known about the upstream regulation of Notch itself. To determine the role of KLF4 in normal (developmental) angiogenesis, we used a mouse retinal angiogenesis model. We found that endothelial-specific overexpression of KLF4 in transgenic mice (EC-K4 Tg) leads to increased vessel density, branching and number of tip cell filopodia as assessed on postnatal day 6 (P6). The hypertrophic vasculature seen with sustained KLF4 overexpression is not stable and undergoes prominent remodeling during P7–P12 resulting in a normal appearing retinal vasculature in adult EC-K4 Tg mice. We find that KLF4 inhibits Delta-like 4 (DLL4) expression in the angiogenic front during retinal vascular development. Furthermore, in an oxygen-induced retinopathy model, overexpression of KLF4 results in decreased vaso-obliteration and neovascular tuft formation that is similar to genetic or pharmacologic DLL4 inhibition. Mechanistically, we show that KLF4 disables the activity of the essential Notch transcriptional activator RBP-J by interfering with binding of co-activators NICD and MAML at intron 3 of the Notch ligand DLL4. In summary, our experimental results demonstrate a regulatory role of KLF4 in developmental angiogenesis through regulation of DLL4 transcription.






Similar content being viewed by others
References
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438(7070):932–936
Benedito R et al (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137(6):1124–1135
Bentley K et al (2014) The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16(4):309–321
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7(9):678–689
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66(10):1631–1646
Hellstrom M et al (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780
Pitulescu ME et al (2017) Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol 19(8):915–927
Li JL et al (2007) Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67(23):11244–11253
Noguera-Troise I et al (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444(7122):1032–1037
Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16(2):196–208
McConnell BB, Yang VW (2010) Mammalian Kruppel-like factors in health and diseases. Physiol Rev 90(4):1337–1381
Bieker JJ (2001) Kruppel-like factors: three fingers in many pies. J Biol Chem 276(37):343558
Tetreault MP, Yang Y, Katz JP (2013) Kruppel-like factors in cancer. Nat Rev Cancer 13(10):701–713
Hamik A et al (2007) Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 282(18):13769–13779
Zhou G et al (2012) Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 122(12):4727–4731
Hale AT et al (2014) Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem 289(17):12016–12028
Pitulescu ME et al (2010) Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat Protoc 5(9):1518–1534
Zudaire E et al (2011) A computational tool for quantitative analysis of vascular networks. PLoS ONE 6(11):e27385
Smith LE et al (1994) Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35(1):101–111
Lobov IB et al (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104(9):3219–3224
Connor KM et al (2009) Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4(11):1565–1573
Gerhardt H et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6):1163–1177
Shatat MA et al (2014) Endothelial Kruppel-like factor 4 modulates pulmonary arterial hypertension. Am J Respir Cell Mol Biol 50(3):647–653
Ribatti D, Nico B, Crivellato E (2011) The role of pericytes in angiogenesis. Int J Dev Biol 55(3):261–268
Sheikh AQ et al (2015) Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med 7(308):308ra159
Davies PF et al (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99(2):315–327
Shankman LS et al (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637
Ehling M et al (2013) Notch controls retinal blood vessel maturation and quiescence. Development 140(14):3051–3061
Baluk P et al (2003) Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 163(5):1801–1815
Stahl A et al (2010) The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 51(6):2813–2826
Scott A, Fruttiger M (2010) Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond) 24(3):416–421
Gu X et al (2002) Effects of sustained hyperoxia on revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 43(2):496–502
Okuno Y et al (2012) Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med 18(8):1208–1216
Lobov IB et al (2011) The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 117(24):6728–6737
Liu ZJ et al (2003) Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23(1):14–25
Hainaud P et al (2006) The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res 66(17):8501–8510
Sacilotto N et al (2013) Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA 110(29):11893–11898
Wythe JD et al (2013) ETS factors regulate Vegf-dependent arterial specification. Dev Cell 26(1):45–58
High FA, Epstein JA (2008) The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9(1):49–61
Ziyad S, Iruela-Arispe ML (2011) Molecular mechanisms of tumor angiogenesis. Genes Cancer 2(12):1085–1096
Swift MR, Weinstein BM (2009) Arterial-venous specification during development. Circ Res 104(5):576–588
Gridley T (2007) Notch signaling in vascular development and physiology. Development 134(15):2709–2718
Sangwung P et al (2017) KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2(4):e91700
Lee TH et al (2006) Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of alpha6beta1 integrin in angiogenesis. J Biol Chem 281(52):40450–40460
Estrach S et al (2011) Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res 109(2):172–182
Seo S, Kume T (2006) Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol 296(2):421–436
Hayashi H, Kume T (2008) Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE 3(6):e2401
Lilly AJ, Lacaud G, Kouskoff V (2017) SOXF transcription factors in cardiovascular development. Semin Cell Dev Biol 63:50–57
Caolo V et al (2010) Feed-forward signaling by membrane-bound ligand receptor circuit: the case of NOTCH DELTA-like 4 ligand in endothelial cells. J Biol Chem 285(52):40681–40689
Diez H et al (2007) Hypoxia-mediated activation of Dll4–Notch–Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res 313(1):1–9
Acknowledgements
We are grateful for the support and encouragement provided by Dr. Mukesh K. Jain. We thank Dr. Christina Antonopoulos-Buzzy (Case Western Reserve University School of Medicine) for kindly providing technical support, discussion, and review of the manuscript. We thank Kevin P. Montgomery for assistance with figures. This work is supported by NIH R01HL113570.
Author information
Authors and Affiliations
Contributions
EB designed, performed, and analyzed experiments, and drafted the manuscript. HZ, JP, and MAS provided input into experimental results and reviewed the manuscript. RHA provided crucial reagents and reviewed the manuscript. AH conceived and coordinated the studies, designed, and analyzed the experiments, and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boriushkin, E., Zhang, H., Becker, M. et al. Kruppel-like factor 4 regulates developmental angiogenesis through disruption of the RBP-J–NICD–MAML complex in intron 3 of Dll4. Angiogenesis 22, 295–309 (2019). https://doi.org/10.1007/s10456-018-9657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9657-y