Abstract
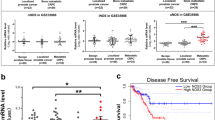
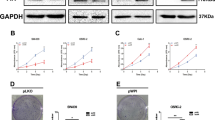
Tissue microarray analysis confirmed higher dimethylarginine dimethylaminohydrolase-1 (DDAH1) expression in prostate cancer (PCa) compared to benign and normal prostate tissues. DDAH1 regulates nitric oxide (NO) production by degrading endogenous nitric oxide synthase (NOS) inhibitor, asymmetric dimethylarginine (ADMA). This study examined whether DDAH1 has any physiological role in PCa progression. Using overexpression of DDAH1 in PCa (PC3 and LNCaP) cell lines, we found that DDAH1 promotes cell proliferation, migration and invasion by lowering ADMA levels, as well as increasing NO production. VEGF, HIF-1α and iNOS were upregulated in DDAH1 expressing cells as result of elevated NO. DDAH1 increased secretion of pro-angiogenic signals bFGF and IL-8, into conditioned media. Treatment of DDAH1-positive PCa cells with NOS inhibitors (L-NAME and 1400 W) attenuated DDAH1 activity to promote cell growth. Xenografts derived from these cells grew significantly faster (> twofold) than those derived from control cells. Proliferation rate of cells stably expressing mutant DDAH1 was same as control cells unlike wild-type DDAH1-positive PCa cells. Xenograft tumors derived from mutant-positive cells did not differ from control tumors. VEGF, HIF-1α and iNOS expression did not differ in DDAH1 mutant-positive tumors compared to control tumors, but was upregulated in wild-type DDAH1 overexpressing tumors. Furthermore, CD31 immunostaining on xenograft tissues demonstrated that DDAH1 tumors had high endothelial content than mutant DDAH1 tumors. These data suggest that DDAH1 is an important mediator of PCa progression and NO/DDAH pathway needs to be considered in developing therapeutic strategies targeted at PCa.







Similar content being viewed by others
Abbreviations
- PCa:
-
Prostate cancer
- DDAH1:
-
Dimethylarginine dimethylaminohydrolase-1
- ADMA:
-
Asymmetric dimethylarginine
- NO:
-
Nitric oxide
References
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64(2):327–336
Fidler IJ, Ellis LM (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79(2):1825–188
Folkman J (1995) Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333(26):1757–1763. https://doi.org/10.1056/NEJM199512283332608
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Chang C-F, Diers AR, Hogg N (2015) Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med 79:324–336. https://doi.org/10.1016/j.freeradbiomed.2014.11.012
Sorrenti V (2011) The DDAH/NOS pathway in human prostatic cancer cell lines: antiangiogenic effect of L-NAME. Int J Oncol 39:1303–1310. https://doi.org/10.3892/ijo.2011.1107
Ziche M (1994) Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance. J Clin Invest 94:2036–2044
Janakiram NB, Rao CV (2012) iNOS-selective inhibitors for cancer prevention: promise and progress. Future Med Chem 4(17):2193–2204. https://doi.org/10.4155/fmc.12.168
Muntane J, la Mata MD (2010) Nitric oxide and cancer. World J Hepatol 2(9):337–344. https://doi.org/10.4254/wjh.v2.i9.337
Vannini F, Kashfi K, Nath N (2015) The dual role of iNOS in cancer. Redox Biol 6:334–343
Leiper J, Nandi M (2011) The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10(4):277–291. https://doi.org/10.1038/nrd3358
Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP (2005) Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation 111(11):1431–1438. https://doi.org/10.1161/01.CIR.0000158487.80483.09
Palm F, Onozato ML, Luo Z, Wilcox CS (2007) Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293(6):H3227–H3245. https://doi.org/10.1152/ajpheart.00998.2007
Kostourou V, Robinson SP, Cartwright JE, Whitley GS (2002) Dimethylarginine dimethylaminohydrolase I enhances tumour growth and angiogenesis. Br J Cancer 87(6):673–680. https://doi.org/10.1038/sj.bjc.6600518
Wang Y, Hu S, Gabisi AM Jr, Er JA, Pope A, Burstein G, Schardon CL, Cardounel AJ, Ekmekcioglu S, Fast W (2014) Developing an irreversible inhibitor of human DDAH-1, an enzyme upregulated in melanoma. Chem Med Chem 9(4):792–797. https://doi.org/10.1002/cmdc.201300557
Ummanni R, Junker H, Zimmermann U, Venz S, Teller S, Giebel J, Scharf C, Woenckhaus C, Dombrowski F, Walther R (2008) Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer Lett 266(2):171–185
Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, Houdijk AP, Geller DA, van Leeuwen PA (2017) A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis. https://doi.org/10.1007/s10456-017-9567-4
Tran CT, Fox MF, Vallance P, Leiper JM (2000) Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 68(1):101–105. https://doi.org/10.1006/geno.2000.6262
Ummanni R, Mundt F, Pospisil H, Venz S, Scharf C, Barett C, Falth M, Kollermann J, Walther R, Schlomm T, Sauter G, Bokemeyer C, Sultmann H, Schuppert A, Brummendorf TH, Balabanov S (2011) Identification of clinically relevant protein targets in prostate cancer with 2D-DIGE coupled mass spectrometry and systems biology network platform. PLoS One 6(2):e16833. https://doi.org/10.1371/journal.pone.0016833
Knipp M, Vasak M (2000) A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 286(2):257–264. https://doi.org/10.1006/abio.2000.4805
Ware JL, DeLong ER (1985) Influence of tumour size on human prostate tumour metastasis in athymic nude mice. Br J Cancer 51(3):419–423
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5(5):402–418. https://doi.org/10.1016/j.apsb.2015.07.005
Monteiro HP, Gruia-Gray J, Peranovich TMS, De Oliveira LCB, Stern A (1999) Nitric oxide stimulates tyrosine phosphorylation of focal adhesion kinase, SRC kinase, and mitogen-activated protein kinases in murine fibroblasts. Free Radic Biol Med 28(2):174–182
Figel S (2011) Focal adhesion kinase controls prostate cancer progression via intrinsic kinase and scaffolding functions. Anti-Cancer Agents Med Chem 11:607–616
Smith CL, Birdsey GM, Anthony S, Arrigoni FI, Leiper JM, Vallance P (2003) Dimethylarginine dimethylaminohydrolase activity modulates ADMA levels, VEGF expression, and cell phenotype. Biochem Biophys Res Commun 308(4):984–989
Hasegawa K, Wakino S, Tanaka T, Kimoto M, Tatematsu S, Kanda T, Yoshioka K, Homma K, Sugano N, Kurabayashi M, Saruta T, Hayashi K (2006) Dimethylarginine dimethylaminohydrolase 2 increases vascular endothelial growth factor expression through Sp1 transcription factor in endothelial cells. Arterioscler Thromb Vasc Biol 26(7):1488–1494. https://doi.org/10.1161/01.ATV.0000219615.88323.b4
Fukumura D, Kashiwagi S, Jain RK (2006) The role of nitric oxide in tumour progression. Nat Rev Cancer 6(7):521–534. https://doi.org/10.1038/nrc1910
Du Q, Zhang X, Liu Q, Zhang X, Bartels CE, Geller DA (2013) Nitric oxide production upregulates Wnt/beta-catenin signaling by inhibiting Dickkopf-1. Cancer Res 73(21):6526–6537. https://doi.org/10.1158/0008-5472.CAN-13-1620
Chang CF, Diers AR, Hogg N (2015) Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med 79:324–336. https://doi.org/10.1016/j.freeradbiomed.2014.11.012
Faton (2002) Role of nitric oxide in the regulation hif1a during hypoxia. Am J Physiol Cell Physiol 283:C178–C186
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20(1):51–56. https://doi.org/10.1016/j.gde.2009.10.009
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10. https://doi.org/10.1159/000088478
Sooriakumaran P, Kaba R (2005) Angiogenesis and the tumour hypoxia response in prostate cancer: a review. Int J Surg 3(1):61–67. https://doi.org/10.1016/j.ijsu.2005.03.013
Ayling LJ, Whitley GS, Aplin JD, Cartwright JE (2006) Dimethylarginine dimethylaminohydrolase (DDAH) regulates trophoblast invasion and motility through effects on nitric oxide. Hum Reprod 21(10):2530–2537. https://doi.org/10.1093/humrep/del111
Varner JA, Cheresh DA (1996) Tumor angiogenesis and the role of vascular cell integrin alphavbeta3. Important Adv Oncol 69–87
Figel S, Gelman IH (2011) Focal adhesion kinase controls prostate cancer progression via intrinsic kinase and scaffolding functions. Anticancer Agents Med Chem 11(7):607–616
Zhao X, Guan JL (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63(8):610–615. https://doi.org/10.1016/j.addr.2010.11.001
Guarino M (2010) Src signaling in cancer invasion. J Cell Physiol 223(1):14–26. https://doi.org/10.1002/jcp.22011
Boult JK, Walker-Samuel S, Jamin Y, Leiper JM, Whitley GS, Robinson SP (2011) Active site mutant dimethylarginine dimethylaminohydrolase 1 expression confers an intermediate tumour phenotype in C6 gliomas. J Pathol 225(3):344–352. https://doi.org/10.1002/path.2904
Trojan L, Thomas D, Friedrich D, Grobholz R, Knoll T, Alken P, Michel MS (2004) Expression of different vascular endothelial markers in prostate cancer and BPH tissue: an immunohistochemical and clinical evaluation. Anticancer Res 24(3a):1651–1656
Kluetz PG, Figg WD, Dahut WL (2010) Angiogenesis inhibitors in the treatment of prostate cancer. Expert Opin Pharmacother 11(2):233–247
Mukherji D, Temraz S, Wehbe D, Shamseddine A (2013) Angiogenesis and anti-angiogenic therapy in prostate cancer. Crit Rev Oncol Hematol 87(2):122–131
Fu W, Madan E, Yee M, Zhang H (2012) Progress of molecular targeted therapies for prostate cancers. Biochim Biophys Acta (BBA) Rev Cancer 1825(2):140–152
Acknowledgements
This work is supported by SMILE (CSC-0111) project supported by Council for Scientific and Industrial Research (CSIR) under 12th five-year plan during 2012 to 2017. KKR acknowledge UGC for CSIR-UGC fellowship for graduate students.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, K.R.K., Dasari, C., Duscharla, D. et al. Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA). Angiogenesis 21, 79–94 (2018). https://doi.org/10.1007/s10456-017-9587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9587-0