Abstract
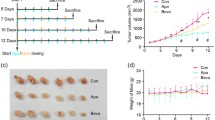
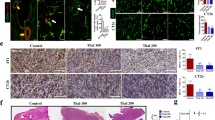
Blockade of the glycolytic activator PFKFB3 in cancer cells (using a maximum tolerable dose of 70 mg/kg of the PFKFB3 blocker 3PO) inhibits tumor growth in preclinical models and is currently being tested as a novel anticancer treatment in phase I clinical trials. However, a detailed preclinical analysis of the effects of such maximum tolerable dose of a PFKFB3 blocker on the tumor vasculature is lacking, even though tumor endothelial cells are hyper-glycolytic. We report here that a high dose of 3PO (70 mg/kg), which inhibits cancer cell proliferation and reduces primary tumor growth, causes tumor vessel disintegration, suppresses endothelial cell growth for protracted periods, (model-dependently) aggravates tumor hypoxia, and compromises vascular barrier integrity, thereby rendering tumor vessels more leaky and facilitating cancer cell intravasation and dissemination. These findings contrast to the effects of a low dose of 3PO (25 mg/kg), which induces tumor vessel normalization, characterized by vascular barrier tightening and maturation, but reduces cancer cell intravasation and metastasis. Our findings highlight the importance of adequately dosing a glycolytic inhibitor for anticancer treatment.




Similar content being viewed by others
References
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20. doi:10.1016/j.cmet.2007.10.002
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7(1):110–120. doi:10.1158/1535-7163.MCT-07-0482
Van Schaftingen E, Lederer B, Bartrons R, Hers HG (1982) A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem 129(1):191–195
Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA 2nd, Klarer AC, Redman R, Miller DM, Trent JO, Telang S, Chesney J (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12(8):1461–1470. doi:10.1158/1535-7163.MCT-13-0097
Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen LA, Schoors S, Boeckx B, Vriens J, Kuchnio A, Veys K, Cruys B, Finotto L, Treps L, Stav-Noraas TE, Bifari F, Stapor P, Decimo I, Kampen K, De Bock K, Haraldsen G, Schoonjans L, Rabelink T, Eelen G, Ghesquiere B, Rehman J, Lambrechts D, Malik AB, Dewerchin M, Carmeliet P (2016) Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30(6):968–985. doi:10.1016/j.ccell.2016.10.006
Cantelmo AR, Pircher A, Kalucka J, Carmeliet P (2017) Vessel pruning or healing: endothelial metabolism as a novel target? Expert Opin Ther Targets 21(3):239–247. doi:10.1080/14728222.2017.1282465
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10(6):417–427. doi:10.1038/nrd3455
Jain RK (2014) Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26(5):605–622. doi:10.1016/j.ccell.2014.10.006
Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P (2009) Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136(5):839–851. doi:10.1016/j.cell.2009.01.020
Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion AC, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquiere B, Annaert W, Agostinis P, Carmeliet P (2014) Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26(2):190–206. doi:10.1016/j.ccr.2014.06.025
Salsbury AJ, Burrage K, Hellmann K (1970) Inhibition of metastatic spread by I.C.R.F. 159: selective deletion of a malignant characteristic. Br Med J 4(5731):344–346
Le Serve AW, Hellmann K (1972) Metastases and the normalization of tumour blood vessels by ICRF 159: a new type of drug action. Br Med J 1(5800):597–601
Hellmann K (2004) Recognition of tumor blood vessel normalization as a new antiangiogenic concept. Nat Med 10 (4):329; author reply 329–330. doi:10.1038/nm0404-329a
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663. doi:10.1016/j.cell.2013.06.037
Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouche A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P (2014) Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19(1):37–48. doi:10.1016/j.cmet.2013.11.008
Clem BF, O’Neal J, Klarer AC, Telang S, Chesney J (2016) Clinical development of cancer therapeutics that target metabolism. QJM 109(6):367–372. doi:10.1093/qjmed/hcv181
Redman R, Pohlmann P, Kurmann M, Tapolsky G, Chesney A (2015) A phase I, dose-escalation, multi-center study of PFK-158 in patients with advanced solid malignancies explores a first-in-man inhbibitor of glycolysis. J Clin Oncol 33(15). doi:10.1200/jco.2015.33.15_suppl.tps2606
Maes H, Van Eygen S, Krysko DV, Vandenabeele P, Nys K, Rillaerts K, Garg AD, Verfaillie T, Agostinis P (2014) BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death Dis 5:e1127. doi:10.1038/cddis.2014.94
Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr, Schabel FM Jr (1984) Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 44(2):717–726
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52(11):2745–2756. doi:10.1172/JCI107470
Korff T, Krauss T, Augustin HG (2004) Three-dimensional spheroidal culture of cytotrophoblast cells mimics the phenotype and differentiation of cytotrophoblasts from normal and preeclamptic pregnancies. Exp Cell Res 297(2):415–423. doi:10.1016/j.yexcr.2004.03.043
Kuchnio A, Moens S, Bruning U, Kuchnio K, Cruys B, Thienpont B, Broux M, Ungureanu AA, Leite de Oliveira R, Bruyere F, Cuervo H, Manderveld A, Carton A, Hernandez-Fernaud JR, Zanivan S, Bartic C, Foidart JM, Noel A, Vinckier S, Lambrechts D, Dewerchin M, Mazzone M, Carmeliet P (2015) The cancer cell oxygen sensor PHD2 promotes metastasis via activation of cancer-associated fibroblasts. Cell Rep 12(6):992–1005. doi:10.1016/j.celrep.2015.07.010
Fan TW, Lorkiewicz PK, Sellers K, Moseley HN, Higashi RM, Lane AN (2012) Stable isotope-resolved metabolomics and applications for drug development. Pharmacol Ther 133(3):366–391. doi:10.1016/j.pharmthera.2011.12.007
Fraccaroli A, Pitter B, Taha AA, Seebach J, Huveneers S, Kirsch J, Casaroli-Marano RP, Zahler S, Pohl U, Gerhardt H, Schnittler HJ, Montanez E (2015) Endothelial alpha-parvin controls integrity of developing vasculature and is required for maintenance of cell–cell junctions. Circ Res 117(1):29–40. doi:10.1161/CIRCRESAHA.117.305818
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131(3):463–475. doi:10.1016/j.cell.2007.08.038
Giannotta M, Trani M, Dejana E (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26(5):441–454. doi:10.1016/j.devcel.2013.08.020
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG (2000) Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 60(5):1388–1393
Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP (2015) A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 6(28):25677–25695. doi:10.18632/oncotarget.4499
Telang S, Clem BF, Klarer AC, Clem AL, Trent JO, Bucala R, Chesney J (2012) Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med 10:95. doi:10.1186/1479-5876-10-95
De Bock K, Mazzone M, Carmeliet P (2011) Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol 8(7):393–404. doi:10.1038/nrclinonc.2011.83
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91(3):1071–1121. doi:10.1152/physrev.00038.2010
Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK (2001) In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 7(7):864–868. doi:10.1038/89997
Acknowledgements
We acknowledge VRC core facilities for their contributions.
Funding
LCC is supported by the Else Kroener-Fresenius-Stiftung and the Fritz Thyssen Stiftung (10.16.2.017MN); AB is supported by an Erasmus Mundus, ERAWEB fellowship (E2.D2.14.289); ARC is supported by the Research Foundation Flanders (FWO 1246617N); AP is supported by an Erwin Schrödinger Fellowship of the Austrian Science Fund FWF (J3730-B26). The work of PC is supported by a Belgian Science Policy Grant (IUAP7/03), long-term structural Methusalem funding by the Flemish Government, Grants from the FWO (G.0834.13N and G.0532.10N), Foundation against Cancer (Grant no. 2012-175), a European Research Council (ERC) Advanced Research Grant (EU ERC269073), and an AXA Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PC declares to be named as inventor on patent applications claiming subject matter related to the findings reviewed in this publication. The other authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Concentration-dependent effects of 3PO on EC glycolysis and survival. A, Concentration–response analysis of the effect of 3PO on proliferation of ECs (n = 3). B, Concentration–response analysis of the effect of 3PO on EC cell death measured by LDH release (n = 3). C, Concentration–response analysis of the effect of 3PO on the glycolytic flux of ECs (n = 3). D, E, Morphometric quantification of EC spheroid sprouting, revealing that blockade of PFKFB3 by the high concentration of 3PO (30 μM) reduced total sprout length (D) and number of sprouts per spheroid (E) more pronouncedly than the low concentration of 3PO (10 μM) (mean ± SEM; n = 10). F–H, Representative bright-field micrographs of control (F) and low-concentration (G, 10 μM) and high-concentration (H, 30 μM) 3PO-treated EC spheroids, showing that vascular sprouting is most impaired by the high concentration of 3PO. Scale bars: 50 μm. All quantitative data are mean ± SEM. * p < 0.05. (PDF 143 kb)
Rights and permissions
About this article
Cite this article
Conradi, LC., Brajic, A., Cantelmo, A.R. et al. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis 20, 599–613 (2017). https://doi.org/10.1007/s10456-017-9573-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9573-6