Abstract
Background
Sex-related differences in the role of androgen have been reported in cardiovascular diseases and angiogenesis. Moreover, androgen receptor (AR) has been causally involved in the homeostasis of human prostate endothelial cells. However, levels of expression, functionality and biological role of AR in male- and female-derived human endothelial cells (ECs) remain poorly characterized. The objectives of this work were (1) to characterize the functional expression of AR in male- and female-derived human umbilical vein endothelial cell (HUVEC), and (2) to specifically analyze the biological effects of DHT, and the role of AR on these effects, in male-derived HUVECs (mHUVECs).
Results
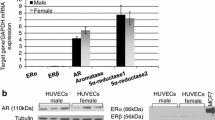
Immunohistochemical analyses of tissue microarrays from benign human tissues confirmed expression of AR in ECs from several androgen-regulated and non-androgen-regulated human organs. Functional expression of AR was validated in vitro in male- and female-derived HUVECs using quantitative RT-PCR, immunoblotting and AR-mediated transcriptional activity assays. Our results indicated that functional expression of AR in male- and female-derived HUVECs was heterogeneous, but not sex dependent. In parallel, we analyzed in depth the biological effects of DHT, and the role of AR on these effects, on proliferation, survival and tube formation capacity in mHUVECs. Our results indicated that DHT did not affect mHUVEC survival; however, DHT stimulated mHUVEC proliferation and suppressed mHUVEC tube formation capacity. While the effect of DHT on proliferation was mediated through AR, the effect of DHT on tube formation did not depend on the presence of a functional AR, but rather depended on the ability of mHUVECs to further metabolize DHT.
Conclusions
(1) Heterogeneous expression of AR in male- and female-derived HUVEC could define the presence of functionally different subpopulations of ECs that may be affected differentially by androgens, which could explain, at least in part, the pleiotropic effects of androgen on vascular biology, and (2) DHT, and metabolites of DHT, generally thought to represent progressively more hydrophilic products along the path to elimination, may have differential roles in modulating the biology of human ECs through AR-dependent and AR-independent mechanisms, respectively.







Similar content being viewed by others
References
Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D (1992) Evolution of the nuclear receptor gene superfamily. EMBO J 11(3):1003–1013
Blauer M, Vaalasti A, Pauli SL, Ylikomi T, Joensuu T, Tuohimaa P (1991) Location of androgen receptor in human skin. J Invest Dermatol 97(2):264–268
Laine M, Blauer M, Ylikomi T, Tuohimaa P, Aitasalo K, Happonen RP, Tenovuo J (1993) Immunohistochemical demonstration of androgen receptors in human salivary glands. Arch Oral Biol 38(4):299–302
Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE (1997) The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82(10):3493–3497
Mantalaris A, Panoskaltsis N, Sakai Y, Bourne P, Chang C, Messing EM, Wu JH (2001) Localization of androgen receptor expression in human bone marrow. J Pathol 193(3):361–366
Schultheiss D, Badalyan R, Pilatz A, Gabouev AI, Schlote N, Wefer J, von Wasielewski R, Mertsching H, Sohn M, Stief CG, Jonas U (2003) Androgen and estrogen receptors in the human corpus cavernosum penis: immunohistochemical and cell culture results. World J Urol 21(5):320–324
Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S (2004) Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89(10):5245–5255
Godoy A, Watts A, Sotomayor P, Montecinos VP, Huss WJ, Onate SA, Smith GJ (2008) Androgen receptor is causally involved in the homeostasis of the human prostate endothelial cell. Endocrinology 149(6):2959–2969. doi:10.1210/en.2007-1078
Godoy AS, Chung I, Montecinos VP, Buttyan R, Johnson CS, Smith GJ (2013) Role of androgen and vitamin D receptors in endothelial cells from benign and malignant human prostate. Am J Physiol Endocrinol Metab 304(11):E1131–E1139. doi:10.1152/ajpendo.00602.2012
Torres-Estay V, Carreno DV, San Francisco IF, Sotomayor P, Godoy AS, Smith GJ (2015) Androgen receptor in human endothelial cells. J Endocrinol 224(3):R131–R137. doi:10.1530/JOE-14-0611
Yoshida S, Ikeda Y, Aihara K (2016) Roles of the androgen–androgen receptor system in vascular angiogenesis. J Atheroscler Thromb 23(3):257–265. doi:10.5551/jat.31047
Buttyan R, Shabsigh A, Perlman H, Colombel M (1999) Regulation of apoptosis in the prostate gland by androgenic steroids. Trends Endocrinol Metab 10(2):47–54
Cheng L, Zhang S, Sweeney CJ, Kao C, Gardner TA, Eble JN (2004) Androgen withdrawal inhibits tumor growth and is associated with decrease in angiogenesis and VEGF expression in androgen-independent CWR22Rv1 human prostate cancer model. Anticancer Res 24(4):2135–2140
Godoy A, Montecinos VP, Gray DR, Sotomayor P, Yau JM, Vethanayagam RR, Singh S, Mohler JL, Smith GJ (2011) Androgen deprivation induces rapid involution and recovery of human prostate vasculature. Am J Physiol Endocrinol Metab 300(2):E263–E275. doi:10.1152/ajpendo.00210.2010
Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, Metter EJ (2006) Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab 290(2):E234–E242
Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C (1999) Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res 84(7):813–819
Dockery F, Bulpitt CJ, Donaldson M, Fernandez S, Rajkumar C (2003) The relationship between androgens and arterial stiffness in older men. J Am Geriatr Soc 51(11):1627–1632
Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS (2003) Testosterone as a protective factor against atherosclerosis–immunomodulation and influence upon plaque development and stability. J Endocrinol 178(3):373–380
Kang SM, Jang Y, Kim JY, Chung N, Cho SY, Chae JS, Lee JH (2002) Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol 89(7):862–864
Kalin MF, Zumoff B (1990) Sex hormones and coronary disease: a review of the clinical studies. Steroids 55(8):330–352
Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A (2007) Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis 18(1):9–13
Oesterling JE (1994) Endocrine therapies for symptomatic benign prostatic hyperplasia. Urology 43(2 Suppl):7–16
Alonzi R, Padhani AR, Taylor NJ, Collins DJ, D’Arcy JA, Stirling JJ, Saunders MI, Hoskin PJ (2011) Antivascular effects of neoadjuvant androgen deprivation for prostate cancer: an in vivo human study using susceptibility and relaxivity dynamic MRI. Int J Radiat Oncol Biol Phys 80(3):721–727
Kravchick S, Cytron S, Mamonov A, Peled R, Linov L (2009) Effect of short-term dutasteride therapy on prostate vascularity in patients with benign prostatic hyperplasia: a pilot study. Urology 73(6):1274–1278
Godoy A, Montecinos VP, Gray DR, Sotomayor P, Yau JM, Vethanayagam RR, Singh S, Mohler JL, Smith GJ (2011) Androgen deprivation induces rapid involution and recovery of human prostate vasculature. Am J Physiol Endocrinol Metab 300(2):E263–E275
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52(11):2745–2756. doi:10.1172/JCI107470
Nachman RL, Jaffe EA (2004) Endothelial cell culture: beginnings of modern vascular biology. J Clin Invest 114(8):1037–1040. doi:10.1172/JCI23284
Sotomayor P, Godoy A, Smith GJ, Huss WJ (2009) Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate 69(4):401–410
Cano P, Godoy A, Escamilla R, Dhir R, Onate SA (2007) Stromal–epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res 67(2):511–519
Shafi AA, Putluri V, Arnold JM, Tsouko E, Maity S, Roberts JM, Coarfa C, Frigo DE, Putluri N, Sreekumar A, Weigel NL (2015) Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget 6(31):31997–32012. doi:10.18632/oncotarget.5585
von der Ahe D, Janich S, Scheidereit C, Renkawitz R, Schutz G, Beato M (1985) Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature 313(6004):706–709
Hsing AW, Reichardt JK, Stanczyk FZ (2002) Hormones and prostate cancer: current perspectives and future directions. Prostate 52(3):213–235
Bishop-Bailey D, Walsh DT, Warner TD (2004) Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA 101(10):3668–3673
He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S (2006) Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98(2):192–199
Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ (2006) The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology 147(9):4025–4033. doi:10.1210/en.2005-1485
Huber RM, Murphy K, Miao B, Link JR, Cunningham MR, Rupar MJ, Gunyuzlu PL, Haws TF, Kassam A, Powell F, Hollis GF, Young PR, Mukherjee R, Burn TC (2002) Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene 290(1–2):35–43
Lobo RA, Speroff L (1994) International consensus conference on postmenopausal hormone therapy and the cardiovascular system. Fertil Steril 61(4):592–595
Milewich L, Kaimal V, Johnson AR (1987) Steroid 5 alpha-reductase activity in endothelial cells from human umbilical cord vessels. J Steroid Biochem 26(5):561–567
Mukherjee TK, Dinh H, Chaudhuri G, Nathan L (2002) Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci USA 99(6):4055–4060
Feldman BJ, Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1(1):34–45
Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, deVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS (1997) Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol 11(4):450–459
Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK (2010) A sex-specific role for androgens in angiogenesis. J Exp Med 207(2):345–352
Antonarakis ES, Luo J (2015) Prostate cancer: AR splice variant dimerization—clinical implications. Nat Rev Urol 12(8):431–433. doi:10.1038/nrurol.2015.184
Leibrand CR, Price DK, Figg WD (2016) Androgen receptor splice variant 7 (AR-V7) and drug efficacy in castration-resistant prostate cancer: biomarker for treatment selection exclusion or inclusion? Cancer Biol Ther. doi:10.1080/15384047.2016.1156274
Yoshida S, Aihara K, Ikeda Y, Sumitomo-Ueda Y, Uemoto R, Ishikawa K, Ise T, Yagi S, Iwase T, Mouri Y, Sakari M, Matsumoto T, Takeyama K, Akaike M, Matsumoto M, Sata M, Walsh K, Kato S (2013) Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation 128(1):60–71. doi:10.1161/CIRCULATIONAHA.113.001533
Montecinos VP, Godoy A, Hinklin J, Vethanayagam RR, Smith GJ (2012) Primary xenografts of human prostate tissue as a model to study angiogenesis induced by reactive stroma. PLoS One 7(1):e29623. doi:10.1371/journal.pone.0029623
Montecinos VP, Morales CH, Fischer TH, Burns S, San Francisco IF, Godoy AS, Smith GJ (2015) Selective targeting of bioengineered platelets to prostate cancer vasculature: new paradigm for therapeutic modalities. J Cell Mol Med 19(7):1530–1537. doi:10.1111/jcmm.12515
Tanner MJ, Welliver RC Jr, Chen M, Shtutman M, Godoy A, Smith G, Mian BM, Buttyan R (2011) Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS One 6(1):e16027. doi:10.1371/journal.pone.0016027
Balk SP, Knudsen KE (2008) AR, the cell cycle, and prostate cancer. Nucl Recept Signal 6:e001
Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS (2011) Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol 300(4):H1210–H1221. doi:10.1152/ajpheart.01210.2010
Das A, Yaqoob U, Mehta D, Shah VH (2009) FXR promotes endothelial cell motility through coordinated regulation of FAK and MMP-9. Arterioscler Thromb Vasc Biol 29(4):562–570
Acknowledgments
This study was supported by the Department of Defense W81XWH-12-1-0341 and FONDECYT Regular (1130051 and 1161115) Grants to A. Godoy and FONDECYT Iniciación (11140255) to P. Sotomayor. V. Torres-Estay was supported by a Ph.D. fellowship from CONICYT. The authors thank Dr. Yusser Olguín from Universidad Andres Bello for the graphic design support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Gary J. Smith and Alejandro S. Godoy have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2016_9525_MOESM1_ESM.tif
Supplementary Figure 1. Cellular distribution of AR in mHUVECs. mHUVECs were stimulated for 24 h to vehicle (ethanol) or 1 nM dihydrotestosterone (DHT). Nuclear (N) and cytoplasmic (C) cellular extracts were prepared using the NE-PER kit (ThermoFisher Scientific). Membranes and nuclei were probed using antibodies against AR. Histone H3 (nuclear) and β-tubulin (cytoplasmic) were used as loading controls (TIFF 5416 kb)
10456_2016_9525_MOESM2_ESM.tif
Supplementary Fig. 2. FXR expression in mHUVECs. (A) RT-PCR analysis of expression of FXR in HUVECs obtained from 4 different donors and derived from female (lanes 1 and 2) and male (lanes 3 and 4) fetuses. HepG2 cells were used as positive control (lane 5). GAPDH was used as loading control. (B) RT-PCR analysis of expression of FXR directed against four different regions of the FXR gene in HUVECs exposed to vehicle (lanes 1) or 1 nM DHT (lanes 2). HepG2 cells were used as positive control (lanes 3). GAPDH was used as loading control (TIFF 6509 kb)
Rights and permissions
About this article
Cite this article
Torres-Estay, V., Carreño, D.V., Fuenzalida, P. et al. Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms. Angiogenesis 20, 25–38 (2017). https://doi.org/10.1007/s10456-016-9525-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-016-9525-6