Abstract
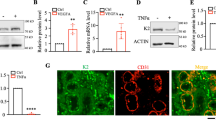
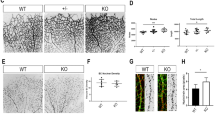
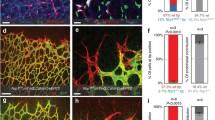
During angiogenesis, endothelial tip cells start sprouting and express delta-like 4 (DLL4) downstream of vascular endothelial growth factor (VEGF). DLL4 subsequently activates Notch in the adjacent stalk cells suppressing sprouting. VEGF also activates A disintegrin and metalloproteases (ADAMs) that induce Notch ectodomain shedding. Although two major ADAMs, i.e. ADAM10 and ADAM17, have been implicated in Notch-signalling activation, their apparent different roles in angiogenesis have not been fully understood yet. The objective of this study was to determine the roles of ADAM10 and ADAM17 activity in angiogenesis. In mouse retinas, ADAM10 or γ-secretase inhibition induced vascular sprouting and density in vivo, whereas attenuation of both ADAM10 and ADAM17 activity produced the opposite phenotype. Retinal blood vessel analysis in ADAM17 hypomorphic mice confirmed the requirement for ADAM17 activity in angiogenesis. However, ADAM17 inhibition did not phenocopy blood vessel increase by Notch blockage. These observations suggest that ADAM17 regulates other fundamental players during angiogenesis besides Notch, which were not affected by ADAM10. By means of an angiogenesis proteome assay, we found that ADAM17 inhibition induced the expression of a naturally occurring inhibitor of angiogenesis Thrombospondin 1 (TSP1), whereas ADAM10 inhibition did not. Accordingly, ADAM17 overexpression downregulated TSP1 expression, and the TSP1 inhibitor LSKL rescued angiogenesis in the tube formation assay downstream of VEGF in the presence of ADAM17 inhibition. Finally, genetic and pharmacological ADAM17 blockade resulted in increased TSP1 expression in mouse retina. Altogether, our results show that ADAM10 and ADAM17 have opposite effects on sprouting angiogenesis that may be unrelated to Notch signalling and involves differentially expressed anti-angiogenic proteins such as TSP1.





Similar content being viewed by others
References
Glomski K, Monette S, Manova K, De Strooper B, Saftig P, Blobel CP (2011) Deletion of Adam10 in endothelial cells leads to defects in organ-specific vascular structures. Blood 118(4):1163–1174
Donners MM, Wolfs IM, Olieslagers S, Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, van Buul JD, Caolo V, Molin DG, Post MJ, Waltenberger J (2010) A disintegrin and metalloprotease 10 is a novel mediator of vascular endothelial growth factor-induced endothelial cell function in angiogenesis and is associated with atherosclerosis. Arterioscler Thromb Vasc Biol 30(11):2188–2195
Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP (2010) Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res 106(5):932–940
Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780
Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA (1998) An essential role for ectodomain shedding in mammalian development. Science 282(5392):1281–1284
van Tetering G, van Diest P, Verlaan I, van der Wall E, Kopan R, Vooijs M (2009) Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J Biol Chem 284(45):31018–31027
Groot AJ, Cobzaru C, Weber S, Saftig P, Blobel CP, Kopan R, Vooijs M, Franzke CW (2013) Epidermal ADAM17 is dispensable for Notch activation. J Invest Dermatol 133(9):2286
Manilay JO, Anderson AC, Kang C, Robey EA (2005) Impairment of thymocyte development by dominant-negative Kuzbanian (ADAM-10) is rescued by the Notch ligand, delta-1. J Immunol 174(11):6732–6741
Weber S, Niessen MT, Prox J, Lullmann-Rauch R, Schmitz A, Schwanbeck R, Blobel CP, Jorissen E, de Strooper B, Niessen CM, Saftig P (2011) The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development 138(3):495–505
Bozkulak EC, Weinmaster G (2009) Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol 29(21):5679–5695
Sulis ML, Saftig P, Ferrando AA (2011) Redundancy and specificity of the metalloprotease system mediating oncogenic NOTCH1 activation in T-ALL. Leukemia 25(10):1564–1569
Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, Hartmann D, Cichy J, Gavrilova O, Schreiber S, Jostock T, Matthews V, Hasler R, Becker C, Neurath MF, Reiss K, Saftig P, Scheller J, Rose-John S (2010) Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med 207(8):1617–1624
Williams CK, Li JL, Murga M, Harris AL, Tosato G (2006) Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 107(3):931–939
Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, Petrovich R, Lipsky PE, Moss ML, Werb Z (2007) The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem 282(20):14836–14844
van den Akker NM, Caolo V, Wisse LJ, Peters PP, Poelmann RE, Carmeliet P, Molin DG, Gittenberger-de Groot AC (2008) Developmental coronary maturation is disturbed by aberrant cardiac vascular endothelial growth factor expression and notch signalling. Cardiovasc Res 78(2):366–375
Sawamiphak S, Ritter M, Acker-Palmer A (2010) Preparation of retinal explant cultures to study ex vivo tip endothelial cell responses. Nat Protoc 5(10):1659–1665
Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME (2007) Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134(18):3317–3326
Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin LE (2009) Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am J Physiol Heart Circ Physiol 296(5):H1344–H1351. doi:10.1152/ajpheart.01246.2008
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15(7):807–817
Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD (2004) Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101(45):15949–15954
Hainaud P, Contreres JO, Villemain A, Liu LX, Plouet J, Tobelem G, Dupuy E (2006) The role of the vascular endothelial growth factor-delta-like 4 ligand/notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res 66(17):8501–8510
Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL (2005) Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 65(19):8690–8697
Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G (2007) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Novartis Found Symp 283:106–120; discussion 121–105, 238–141
Thurston G, Noguera-Troise I, Yancopoulos GD (2007) The Delta paradox: dLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 7(5):327–331
Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD (2010) Chronic DLL4 blockade induces vascular neoplasms. Nature 463(7282):E6–E7
Das S, Czarnek M, Bzowska M, Mezyk-Kopec R, Stalinska K, Wyroba B, Sroka J, Jucha J, Deneka D, Stoklosa P, Ogonek J, Swartz MA, Madeja Z, Bereta J (2012) ADAM17 silencing in mouse colon carcinoma cells: the effect on tumoricidal cytokines and angiogenesis. PLoS One 7(12):e50791
Acknowledgments
We thank Drs. Lorraine Bray from GlaxoSmithKline for the inhibitors GI254023X and GW413333X. We are grateful to Arjan. J. Groot for technical support. The authors gratefully acknowledge the support of the PENT and the CTMM Eminence Programs of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science. M.Vooijs is supported by ERC-Starting grant 208259. A. Chalaris and S. Rose-John are supported by the Deutsche Forschungsgemeinschaft, Bonn (SFB877, project A1) and the cluster of excellence “inflammation and interfaces”. D.G.M. Molin is supported by European grants Interreg IVa Euregio Meuse Rhine BioMIMedics and Interreg IVa Flanders—The Netherlands VaRiA.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standard
All of the reported work has been carried out according to applicable laws and regulations.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Vooijs and M. J. Post have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Caolo, V., Swennen, G., Chalaris, A. et al. ADAM10 and ADAM17 have opposite roles during sprouting angiogenesis. Angiogenesis 18, 13–22 (2015). https://doi.org/10.1007/s10456-014-9443-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-014-9443-4