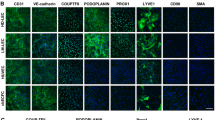
Abstract
Human lymphatic vascular malformations (LMs), also known as cystic hygromas or lymphangioma, consist of multiple lymphatic endothelial cell-lined lymph-containing cysts. No animal model of this disease exists. To develop a mouse xenograft model of human LM, CD34NegCD31Pos LM lymphatic endothelial cells (LM-LEC) were isolated from surgical specimens and compared to foreskin CD34NegCD31Pos lymphatic endothelial cells (LECs). Cells were implanted into a mouse tissue engineering model for 1, 2 and 4 weeks. In vitro LM-LECs showed increased proliferation and survival under starvation conditions (P < 0.0005 at 48 h, two-way ANOVA), increased migration (P < 0.001, two-way ANOVA) and formed fewer (P = 0.029, independent samples t test), shorter tubes (P = 0.029, independent samples t test) than foreskin LECs. In vivo LM-LECs implanted into a Matrigel™-containing mouse chamber model assembled to develop vessels with dilated cystic lumens lined with flat endothelium, morphology similar to that of clinical LMs. Human foreskin LECs failed to survive implantation. In LM-LEC implanted chambers the percent volume of podoplaninPos vessels was 1.18 ± 2.24 % at 1 week, 6.34 ± 2.68 % at 2 weeks and increasing to 7.67 ± 3.60 % at 4 weeks. In conclusion, the significantly increased proliferation, migration, resistance to apoptosis and decreased tubulogenesis of LM-LECs observed in vitro is likely to account for their survival and assembly into stable LM-like structures when implanted into a mouse vascularised chamber model. This in vivo xenograft model will provide the basis of future studies of LM biology and testing of potential pharmacological interventions for patients with lymphatic malformations.





Similar content being viewed by others
References
Brice G, Child AH, Evans A, Bell R, Mansour S, Burnand K, Sarfarazi M, Jeffery S, Mortimer P (2005) Milroy disease and the VEGFR-3 mutation phenotype. J Med Genet 42:98–102
Nakamura K, Rockson SG (2007) Biomarkers of lymphatic function and disease: state of the art and future directions. Mol Diagn Ther 11:227–238
Castro EC, Galambos C (2009) Prox-1 and VEGFR3 antibodies are superior to D2-40 in identifying endothelial cells of lymphatic malformations-a proposal of a new immunohistochemical panel to differentiate lymphatic from other vascular malformations. Pediatr Dev Pathol 12:187–194
Filston HC (1994) Hemangiomas, cystic hygromas, and teratomas of the head and neck. Semin Pediatr Surg 3:147–159
Rottem S, Bronshtein M (1990) Transvaginal sonographic diagnosis of congenital anomalies between 9 weeks and 16 weeks menstrual age. J Clin Ultrasound 18:307–314
Puig S, Casati B, Staudenherz A, Paya K (2005) Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol 53:35–45
Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 69:412–422
Enjolras O, Wassef M, Chapot R (2007) Colour atlas of vascular tumours and vascular malformations. Cambridge University Press, New York
Vikkula M, Boon LM, Mulliken JB, Olsen BR (1998) Molecular basis of vascular anomalies. Trends Cardiovasc Med 8:281–292
Padwa BL, Hayward PG, Ferraro NF, Mulliken JB (1995) Cervicofacial lymphatic malformation: clinical course, surgical intervention, and pathogenesis of skeletal hypertrophy. Plast Reconstr Surg 95:951–960
Whimster IW (1976) The pathology of lymphangioma circumscriptum. Br J Dermatol 94:473–486
Mulliken JB, Glowacki J (1982) Classification of pediatric vascular lesions. Plast Reconstr Surg 70:120–121
Galambos C, Nodit L (2005) Identification of lymphatic endothelium in pediatric vascular tumors and malformations. Pediatr Dev Pathol 8:181–189
Edwards PD, Rahbar R, Ferraro NF, Burrows PE, Mulliken JB (2005) Lymphatic malformation of the lingual base and oral floor. Plast Reconstr Surg 115:1906–1915
Brouillard P, Vikkula M (2003) Vascular malformations: localized defects in vascular morphogenesis. Clin Genet 63:340–351
Huang H-Y, Ho C-C, Huang P-H, Hsu S-M (2001) Co-Expression of VEGF-C and its receptors, VEGFR-2 and VEGFR-3, in endothelial cells of lymphangioma. Implication in autocrine or paracrine regulation of lymphangioma. Lab Invest 81:1729–1734
Jackson IT, Carreno R, Potparic Z, Hussain K (1993) Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg 91:1216–1230
Hitchcock T, Niklason L (2008) Lymphatic tissue engineering: progress and prospects. Ann N Y Acad Sci 1131:44–49
Lokmic Z, Mitchell GM (2008) Engineering the microcirculation. Tissue Eng Part B Rev 14:87–103
Cronin KJ, Messina A, Knight KR, Cooper-White JJ, Stevens GW, Penington AJ, Morrison WA (2004) New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast Reconstr Surg 113:260–269
Tilkorn DJ, Davies EM, Keramidaris E, Dingle AM, Gerrand Y-W, Taylor CJ, Han X-L, Palmer JA, Penington AJ, Mitchell CA, Morrison WA, Dusting GJ, Mitchell GM (2012) The in vitro preconditioning of myoblasts to enhance subsequent survival in an in vivo tissue engineering chamber model. Biomaterials 33:3868–3879
Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438:946–953
Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED (2005) Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate 65:222–230
Zeng Y, Wang F, Williams ED, Chow CW (2005) Lymphatics in the alimentary tract of children in health and disease: study on mucosal biopsies using the monoclonal antibody d2-40. Pediatr Dev Pathol 8:541–549
Zeng Y, Opeskin K, Goad J, Williams ED (2006) Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer Res 66:9566–9575
Coso S, Zeng Y, Opeskin K, Williams ED (2012) Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS ONE 7(6):e39558. doi:10.1371/journal.pone.0039558
Louis KS, Siegel AC (2011) Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol 740:7–12
Howard CV, Reed MG (2005) Unbiased stereology. BIOS Scientific Publishers, Taylor & Francis Group, Oxon
Wilting J, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rössler J (2002) The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J 16:1271–1273
Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21:1299–1308
Swetman GL, Berk DR, Vasanawala SS, Feinstein JA, Lane AT, Bruckner AL (2012) Sildenafil for severe lymphatic malformations. N Engl J Med 366:384–386
Dai Y, Hou F, Saad A, Fan CY, Buckmiller LM, Suen JY, Richter GT (2011) Preliminary investigation of human lymphatic malformations in vitro. Laryngoscope 121:2435–2442
Sun Y, Jia J, Zhang W, Liu B, Zhang Z, Zhao Y (2011) A reproducible in vivo model of lymphatic malformation in rats. J Comp Pathol 145:390–398
Bennett G, April M, Huvos AG (2004) Lymphatic malformation of the sphenoid sinus in a pediatric patient. Otolaryngol Head Neck Surg 131:784–786
Zhang XD, Gillespie SK, Hersey P (2004) Staurosporine induces apoptosis of melanoma by both caspase-dependent and caspase-independent apoptotic pathways. Mol Cancer Ther 2004(3):187–197
Cavallaro U, Dejana E (2011) Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol 12:189–197
Dejana E (2004) Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Raymond MA, Vigneault N, Luyckx V, Hebert MJ (2002) Paracrine repercussions of preconditioning on angiogenesis and apoptosis of endothelial cells. Biochem Biophys Res Commun 291:261–269
Wautier MP, Boval B, Chappey O, Enjolras O, Wernert N, Merland JJ, Wautier JL (1999) Cultured endothelial cells from human arteriovenous malformations have defective growth regulation. Blood 94:2020–2028
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA (2005) PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 19:397–410
Pflicke H, Sixt M (2009) Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 206:2925–2935
Mikhaylova M, Mori N, Wildes FB, Walczak P, Gimi B, Bhujwalla ZM (2008) Hypoxia increases breast cancer cell-induced lymphatic endothelial cell migration. Neoplasia 10:380–389
Garaffa E, Caprara V, Di Castro V, Rosano L, Bagnato A, Spinella F (2012) Endothelin-1 cooperates with hypoxia to induce vascular-like structures through vascular endothelial growth factors -C, -D and -A in lymphatic endothelial cells. Life Sci 19:638–643
Irigoyen M, Anso E, Martinez E, Garoya M, Martinez-Irujo JJ, Rouzant A (2007) Hypoxia alters the adhesive properties of lymphatic endothelial cells. A transcriptional and functional study. Biochim Biophys Acta 1773:880–890
Williams SP, Karnezis T, Achen MG, Stacker SA (2010) Targeting lymphatic vessel functions through tyrosine kinases. J Angiogenes Res 2:13
Folkman J, Kaipainen A (2004) Genes tell lymphatics to sprout or not. Nat Immunol 5:11–12
Norgall S, Papoutsi M, Rossler J, Schweigerer L, Wilting J, Weich HA (2007) Elevated expression of VEGFR-3 in lymphatic endothelial cells from lymphangiomas. BMC Cancer 7:105–122
Stacker S, Hughes R, Williams R, Achen M (2006) Current strategies for modulating lymphangiogenesis signalling pathways in human disease. Curr Med Chem 13:783–792
Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L (2003) Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem 278:40973–40979
Acknowledgments
We thank Michelle Marchionna for technical assistance in the early stages of this project. This work was supported by grants from The National Health and Medical Research Council (NHMRC #566886), The Hector Waldron Pride Charitable Trust (EDW, AJP) and The William Buckland Foundation (AJP, MM). EDW was supported by an NHMRC Career Development Award (#519539). The O’Brien Institute and Monash Institute of Medical Research acknowledge support from the Victorian State Government’s Department of Innovation, Industry and Regional Development’s Operational Infrastructure Support Program.
Conflict of interest
There are no conflicts of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2013_9371_MOESM1_ESM.tif
Expression of CD34 in the lymphatic malformation human tissue. A CD34 expression is not detected in the lymphatic malformation endothelium (#). CD34 is present on the stromal cells in the connective tissue, in cells residing in tunica adventitia of blood vessels and in the endothelium of blood vessels (arrow). B The absence of CD34 from the lymphatic malformation (#) was further confirmed with double labelling of CD34 and CD31 where co-expression of the two is found in blood vessels only (arrow) (TIFF 40192 kb)
10456_2013_9371_MOESM2_ESM.tif
Deposition of connective tissue in the LM xenograft model. Masson’s trichrome stain labels connective tissue component collagen blue (arrows). A, B Connective tissue in 1 week LEC-implanted mouse chamber at low and high magnification respectively. C, D Connective and adipose tissue in 4-week LEC implanted chambers low and high magnification respectively. Note that collagen is most evident at tissue periphery. E, F Connective tissue in 1 week LM-LEC implanted mouse chamber low and high magnification respectively. G, H Connective and adipose tissue in 4-week LM-LEC implanted chambers at low and high magnification respectively. Arrows point to connective tissue in the vicinity of LM vessels (≠). Host artery (#) and vein (*) are present in the chambers (TIFF 50945 kb)
10456_2013_9371_MOESM3_ESM.tif
Cell Proliferation in LM-LEC implanted chambers. A Ki-67 positive proliferating cells were identified in human lymph node, positive control tissue. B, C, D Rare Ki-67-labelled cells were identified in lymphatic malformation vessels at 4 weeks (*). E Ki67 labelled cells were also identified in the host tissue and F in the vicinity of the LM vessels (*). Note variable degree of Ki-67 staining indicative of cell entry or exit from cell proliferation cycle (TIFF 26008 kb)
10456_2013_9371_MOESM4_ESM.tif
Cell death in LM-LEC implanted chambers. A Cleaved caspase-3 positive cells (arrowheads) were identified in human lymph node, positive control tissue. Note different staining patterns within the lymph node follicle. B Cleaved caspase-3 positive cells (arrowheads) were present at the edge of the chamber tissue at 4 weeks and C in the developing host tissue in 1 and 2-week old chambers. D, E Both LEC and LM-LEC implanted chambers contain dead LEC and LM-LECs in the Matrigel where weak residual caspase-3 staining was observed (arrowheads). Note refractive brown dynabead® in the tissue section shown in E. F Hematoxylin stained apoptotic bodies (arrowheads) were evident in both the LEC and LM-LEC implanted chambers in haematoxylin-eosin stained tissues (TIFF 25329 kb)
Rights and permissions
About this article
Cite this article
Lokmic, Z., Mitchell, G.M., Koh Wee Chong, N. et al. Isolation of human lymphatic malformation endothelial cells, their in vitro characterization and in vivo survival in a mouse xenograft model. Angiogenesis 17, 1–15 (2014). https://doi.org/10.1007/s10456-013-9371-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9371-8