Abstract
Introduction
Vascular involvement is a key feature of Systemic sclerosis (SSc). Although the pericytes/endothelial cells (ECs) cross-talk regulates vessels formation, no evidences about the pericytes contribution to ineffective angiogenesis in SSc are available. Recent findings showed similarities between pericytes and Bone Marrow Mesenchymal Stem Cells (BM-MSCs). Due to difficulties in pericytes isolation, this work explores the possibility to use BM-MSCs as pericytes surrogate, clarifying their role in supporting neo-angiogenesis during SSc.
Methods
To demonstrate their potential to normally differentiate into pericytes, both SSc and healthy controls (HC) BM-MSCs were treated with TGF-β and PDGF-BB. The expression of pericytes specific markers (α-SMA, NG2, RGS5 and desmin) was assessed by qPCR, western blot, and immunofluorescence; chemioinvasion and capillary morphogenesis were also performed. Cell-sorting of BM-MSCs co-cultured with HC-ECs was used to identify a possible change in contractile proteins genes expression.
Results
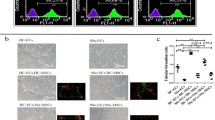
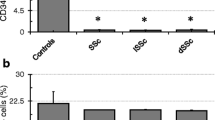
We showed that BM-MSCs isolated from SSc patients displayed an up-regulation of α-SMA and SM22α genes and a reduced proliferative activity. Moreover during SSc, both TGF-β and PDGF-BB can specifically modulate BM-MSCs toward pericytes. TGF-β was found interfering with the PDGF-BB effects. Using BM-MSCs/MVECs co-culture system we observed that SSc BM-MSCs improve ECs tube formation in stressed condition, and BM-MSCs, sorted after co-culture, showed a reduced α-SMA and SM22α gene expression.
Conclusions
BM-MSCs from SSc patients behave as pericytes. They display a more mature and myofibroblast-like phenotype, probably related to microenvironmental cues operating during the disease. After their co-culture with HC-MVECs, SSc BM-MSCs underwent to a phenotypic modulation which re-programs these cells toward a pro-angiogenic behaviour.





Similar content being viewed by others
References
LeRoy EC (1996) Systemic sclerosis. A vascular perspective. Rheum Dis Clin North Am 22:675–694
Kahaleh MB (2004) Vascular involvement in systemic sclerosis (SSc). Clin Exp Rheumatol 22(3 Suppl 33): S19–S23
Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM (2009) Is scleroderma a vasculopathy? Curr Rheumatol Rep 11:103–110
Cipriani P, Guiducci S, Miniati I, Cinelli M, Urbani S et al (2007) Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells:new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum 56:1994–2004
Manetti M, Ibba-Manneschi L, Liakouli V, Guiducci S, Milia AF et al (2010) The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann Rheum Dis 69:598–605
Cipriani P, Franca Milia A, Liakouli V, Pacini A, Manetti M et al (2006) Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic implications. Arthritis Rheum 54:3022–3033
Cipriani P, Marrelli A, Liakouli V, Di Benedetto P, Giacomelli R (2011) Cellular players in angiogenesis during the course of systemic sclerosis. Autoimmun Rev 10:641–646
Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P et al (2011) Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev 10:590–594
Murakami M, Simons M (2009) Regulation of vascular integrity. J Mol Med 87:571–582
Simons M (2005) Angiogenesis: where do we stand now? Circulation 111:1556–1566
Hirschi KK, D’Amore PA (1996) Pericytes in the microvasculature. Cardiovasc Res 32:687–698
Antonelli-Orlidge A, Smith SR, D’Amore PA (1989) Influence of pericytes on capillary endothelial cell growth. Am Rev Respir Dis 140:1129–1131
Gerhardt H, Semb H (2008) Pericytes: gatekeepers in tumour cell metastasis? J Mol Med 86:135–144
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Takakura N (2011) Role of intimate interactions between endothelial cells and the surrounding accessory cells in the maturation of blood vessels. J Thromb Haemost 9:144–150
Hirschi KK, Rohovsky SA, D’Amore PA (1998) PDGF, TGF-beta, and heterotypic cell–cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814
Walshe TE (2010) TGF-beta and microvessel homeostasis. Microvasc Res 80:166–173
Darland DC, D’Amore PA (2001) TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4:11–20
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 11:301–313
da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299
Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I et al (2009) Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev 5:437–445
Bryan BA, D’Amore PA (2008) Pericyte isolation and use in endothelial/pericyte coculture models. Methods Enzymol 443:315–331
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S et al (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 19:324–336
Tormin A, Li O, Brune JC, Walsh S, Schütz B et al (2011) CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 12:5067–5077
da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI (2009) MSC frequency correlates with blood vessel in equine adipose tissue. Tissue Eng Part A 15:221–229
Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A et al (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625–13630
Díaz-Flores L Jr, Madrid JF, Gutiérrez R, Varela H, Valladares F (2006) Adult stem and transit-amplifying cell location. Histol Histopathol 21:995–1027
Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK (2006) Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol 26:2579–2581
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75:487–517
Mack CP (2011) Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31:1495–1505
Challier JC, Kacemi A, Olive G (1995) Mixed culture of pericytes and endothelial cells from fetal microvessels of the human placenta. Cell Mol Biol 41:233–241
Helmbold P, Nayak RC, Marsch WC, Herman IM (2001) Isolation and in vitro characterization of human dermal microvascular pericytes. Microvasc Res 61:160–165
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Hungerford JE, Little CD (1999) Developmental biology of the vascular smooth muscle cell. J Vasc Res 36:2–27
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801
Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I et al (1997) Differentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation 96:82–90
Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N et al (1996) Vascular remodelling. Hypertension 28(3):505–506
Helmbold P, Fiedler E, Fischer M, Marsch WCh (2004) Hyperplasia of dermal microvascular pericytes in scleroderma. J Cutan Pathol 31:431–440
Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM (1999) Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum 42:930–941
Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, et al (2008) Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One 3(1):e 14–52. doi: 10.1371/journal.pone.0001452
Witmer AN, van Blijswijk BC, van Noorden CJF, Vrensen GFJM, Schlingemann RO (2004) In vivo angiogenic phenotype of endothelial cells and pericyte induced by vascular endothelial growth factor-A. J Histochem Cytochem 52:39–52
Palumbo R, Gaetano C, Melillo G, Toschi E, Remuzzi A et al (2000) Shear stress downregulation of platelet-derived growth factor receptor-beta and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation 102:225–230
Newby AC (2006) Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res 69:614–624
Risinger GM Jr, Hunt TS, Updike DL, Bullen EC, Howard EW (2006) Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Biol Chem 281:25915–25925
Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C et al (2008) PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res 23:1519–1528
Varga J, Pasche B (2009) Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5(4):200–206
Hunzelmann N, Brinckmann J (2010) What are the new milestones in the pathogenesis of Systemic Sclerosis? Ann Rheum Dis 69:52–56
von Tell D, Armulik A, Betsholtz C (2006) Pericytes and vascular stability. Exp Cell Res 312(5):623–629
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217(2):318–324
Rouwkema J, de Boer J, Van Blitterswijk CA (2006) Endothelial cells assemble into a 3-dimensional provascular network in a bone tissue engineering construct. Tissue Eng 12(9):2685–2693
Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M (2010) Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res 88:395–405
Acknowledgments
This work was supported by FIRA (Fondazione Italiana Ricerca per l’Artrite) 2009. The authors thank Prof Patricia D’Amore and Dr Tony E. Walshe for their technical assistance in 3D co-culture systems, Dr Maria Paola Nanni Costa and Dr Samuele Di Giovanni for their contribution in BM aspiration.
Ethical standards
The experiments comply with the current laws of Italy.
Conflict of interest
The authors disclose no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cipriani, P., Marrelli, A., Benedetto, P.D. et al. Scleroderma Mesenchymal Stem Cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis 16, 595–607 (2013). https://doi.org/10.1007/s10456-013-9338-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9338-9