Abstract
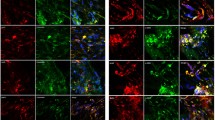
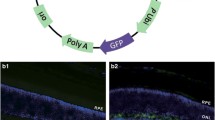
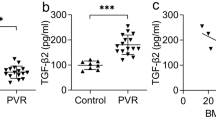
Bone morphorgenetic protein (BMP)-4 has been shown to play a pivotal role in eye development; however, its role in mature retina or ocular angiogenic diseases is unclear. Activating downstream Smad signaling, BMP4 can be either pro-angiogenic or anti-angiogenic, depending on the context of cell types and associated microenvironment. In this study, we generated transgenic mice over-expressing BMP4 in retinal pigment epithelial (RPE) cells (Vmd2-Bmp4 Tg mice), and used the laser-induced choroidal neovascularization (CNV) model to study the angiogenic properties of BMP4 in adult eyes. Vmd2-Bmp4 Tg mice displayed normal retinal histology at 10 weeks of age when compared with age-matched wildtype mice. Over-expression of BMP4 in RPE in the transgenic mice was confirmed by real-time PCR and immunostaining. Elevated levels of Smad1,5 phosphorylation were found in BMP4 transgenic mice compared to wildype mice. Over-expression of BMP4 was associated with less severe CNV as characterized by fluorescein angiography, CNV volume measurement and histology. While control mice showed increased levels of vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP)-9 after laser injury, Vmd2-Bmp4 Tg showed no increase in either VEGF or MMP-9. Further, we found that TNF-induced MMP-9 secretion in vitro was reduced by pretreatment of RPE cells with BMP4. The inhibition of MMP-9 was Smad-dependent because BMP4 failed to repress TNF-induced MMP-9 expression when Smad1,5 was silenced by siRNA. In summary, our studies identified an anti-angiogenic role for BMP4 in laser-induced CNV, mediated by direct inhibition of MMP-9 and indirect inhibition of VEGF.







Similar content being viewed by others
References
Hogan BL (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10(13):1580–1594
Furuta Y, Hogan BL (1998) BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12(23):3764–3775
Muller F, Rohrer H, Vogel-Hopker A (2007) Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development 134(19):3483–3493
Wordinger RJ, Clark AF (2007) Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med 232(8):979–992
Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR (2009) BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem 284(14):9529–9539
Campochiaro PA (2000) Retinal and choroidal neovascularization. J Cell Physiol 184(3):301–310
Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR (1996) Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 37(5):855–868
Zhu D, Deng X, Xu J, Hinton DR (2009) What determines the switch between atrophic and neovascular forms of age related macular degeneration?—the role of BMP4 induced senescence. Aging 1(8):740–745
Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE (2010) The mouse retina as an angiogenesis model. Investig Ophthalmol Vis Sci 51(6):2813–2826
Ryan SJ (1979) The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc 77:707–745
Ferrara N (2010) Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med 16(10):1107–1111
Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA (1997) Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 151(1):281–291
Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer TN, Poulaki V, Ma JJ, Redmond TM, Liu S, Adamis AP, D’Amato RJ (2001) Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol 158(3):1161–1172
Grossniklaus HE, Kang SJ, Berglin L (2010) Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res 29(6):500–519
Garcia-Layana A, Vasquez G, Salinas-Alaman A, Moreno-Montanes J, Recalde S, Fernandez-Robredo P (2009) Development of laser-induced choroidal neovascularization in rats after retinal damage by sodium iodate injection. Ophthal Res 42(4):205–212
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85(3):845–881
Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR (2006) Sequential induction of angiogenic growth factors by TNF-alpha in choroidal endothelial cells. J Neuroimmunol 171(1–2):45–56
Shi X, Semkova I, Muther PS, Dell S, Kociok N, Joussen AM (2006) Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res 83(6):1325–1334
Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T, Werb Z, Baker A, Libert C, Krell HW, Foidart JM, Noel A, Rakic JM (2003) MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. Faseb J 17(15):2290–2292
Hoffmann S, He S, Ehren M, Ryan SJ, Wiedemann P, Hinton DR (2006) MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina 26(4):454–461
Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic JM (2002) Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol 161(4):1247–1253
Xu J, Zhu D, He S, Spee C, Ryan SJ, Hinton DR (2011) Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. Faseb J 25(7):2221–2233
Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK (2007) Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 26(28):4158–4170
Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M (2008) BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res 103(8):804–812
Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, Patterson C, Moser M (2007) ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res 76(3):390–399
Kiyono M, Shibuya M (2003) Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol 23(13):4627–4636
Kiyono M, Shibuya M (2006) Inhibitory Smad transcription factors protect arterial endothelial cells from apoptosis induced by BMP4. Oncogene 25(54):7131–7137
Iacovelli J, Zhao C, Wolkow N, Veldman P, Gollomp K, Ojha P, Lukinova N, King A, Feiner L, Esumi N, Zack DJ, Pierce EA, Vollrath D, Dunaief JL (2011) Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Investig Ophthalmol Vis Sci 52(3):1378–1383
Kachi S, Binley K, Yokoi K, Umeda N, Akiyama H, Muramatu D, Iqball S, Kan O, Naylor S, Campochiaro PA (2009) Equine infectious anemia viral vector-mediated codelivery of endostatin and angiostatin driven by retinal pigmented epithelium-specific VMD2 promoter inhibits choroidal neovascularization. Hum Gene Ther 20(1):31–39
Marmorstein AD, Cross HE, Peachey NS (2009) Functional roles of bestrophins in ocular epithelia. Prog Retin Eye Res 28(3):206–226
Espinosa-Heidmann DG, Suner I, Hernandez EP, Frazier WD, Csaky KG, Cousins SW (2002) Age as an independent risk factor for severity of experimental choroidal neovascularization. Investig Ophthalmol Vis Sci 43(5):1567–1573
Dot C, Parier V, Behar-Cohen F, Benezra D, Jonet L, Goldenberg B, Picard E, Camelo S, de Kozak Y, May F, Soubrane G, Jeanny JC (2009) Influence of age on retinochoroidal healing processes after argon photocoagulation in C57bl/6j mice. Mol Vis 15:670–684
Esumi N, Oshima Y, Li Y, Campochiaro PA, Zack DJ (2004) Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation. J Biol Chem 279(18):19064–19073
Zhou J, Pham L, Zhang N, He S, Gamulescu MA, Spee C, Ryan SJ, Hinton DR (2005) Neutrophils promote experimental choroidal neovascularization. Mol Vis 11:414–424
Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR (2009) A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc 4(5):662–673
Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR (2010) alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 115(16):3398–3406
Ferrara N (2009) VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw 20(4):158–163
Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A (2007) Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci 48(9):4360–4367
Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW (2001) Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet 2(1):18
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA (2009) An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA 106(44):18751–18756
Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ (2003) Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol 197(3):453–462
Vogt RR, Unda R, Yeh LC, Vidro EK, Lee JC, Tsin AT (2006) Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem 98(5):1196–1202
Sonoda S, Sreekumar PG, Kase S, Spee C, Ryan SJ, Kannan R, Hinton DR (2009) Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging 2(1):28–42
Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS (2009) Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun 385(2):198–203
Otto TC, Bowers RR, Lane MD (2007) BMP-4 treatment of C3H10T1/2 stem cells blocks expression of MMP-3 and MMP-13. Biochem Biophys Res Commun 353(4):1097–1104
Zode GS, Clark AF, Wordinger RJ (2009) Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia 57(7):755–766
Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, St-Pierre Y (2002) Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem 277(38):35150–35155
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A (2010) Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39(3):373–384
Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K (2010) miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet 42(12):1101–1108
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Xu and Danhong Zhu contributed equally.
Rights and permissions
About this article
Cite this article
Xu, J., Zhu, D., Sonoda, S. et al. Over-expression of BMP4 inhibits experimental choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis 15, 213–227 (2012). https://doi.org/10.1007/s10456-012-9254-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9254-4