Abstract
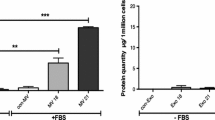
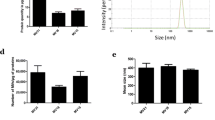
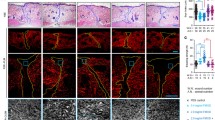
During wound healing, angiogenesis plays a crucial role in inducing adequate perfusion of the new tissue, thereby allowing its survival. This angiogenic process contributes to the formation of granulation tissue, alongside myofibroblasts. Myofibroblasts are cells specialized in wound contraction and synthesis of new extracellular matrix. Fibroblasts, considered by some to be at the origin of myofibroblasts, have already been shown to promote neovascularization. Thus, we hypothesized that myofibroblasts play a key role during angiogenic development in wound healing. We isolated myofibroblasts from normal human skin wounds and dermal microvascular endothelial cells (HDMVEC) and fibroblasts from skin. Using an in vitro fibrin-based model, we compared the proangiogenic activity of wound myofibroblasts to that of fibroblasts in the presence of HDMVEC. By immunostaining with collagen IV antibodies, we observed the formation of a capillary network significantly more developed when HDMVEC were cultured with myofibroblasts compared to the network formed in the presence of fibroblasts. The differences between these cell types did not result from a differential secretion of Vascular Endothelial Growth Factor or basic Fibroblast Growth Factor. However, in the presence of myofibroblasts, a significant decrease in matrix metalloproteinase activity was observed. This finding was correlated with a significant increase in Tissue Inhibitor of MetalloProteinase (TIMP)-1 and TIMP-3. Furthermore, inhibition of TIMP-1 secretion using shRNA significantly decreased myofibroblasts induced angiogenesis. These results led to the hypothesis that normal wound myofibroblasts contribute to the vascular network development during wound healing. Our data emphasize the critical role of wound myofibroblasts during healing.







Similar content being viewed by others
References
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Natl Rev Mol Cell Biol 3(5):349–363
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Desmouliere A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13(1):7–12
Toriseva M, Kahari VM (2009) Proteinases in cutaneous wound healing. Cell Mol Life Sci 66(2):203–224
Villaschi S, Nicosia RF (1994) Paracrine interactions between fibroblasts and endothelial cells in a serum-free coculture model. Modulation of angiogenesis and collagen gel contraction. Lab Invest 71(2):291–299
Velazquez OC, Snyder R, Liu ZJ, Fairman RM, Herlyn M (2002) Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. Faseb J 16(10):1316–1328
Liu H, Chen B, Lilly B (2008) Fibroblasts potentiate blood vessel formation partially through secreted factor TIMP-1. Angiogenesis 11(3):223–234
Chintalgattu V, Nair DM, Katwa LC (2003) Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J Mol Cell Cardiol 35(3):277–286
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348
Fujiwara M, Muragaki Y, Ooshima A (2005) Upregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activity. Arch Dermatol Res 297(4):161–169
Rockey DC, Chung JJ (1996) Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest 98(6):1381–1388
Shahar I, Fireman E, Topilsky M, Grief J, Schwarz Y, Kivity S, Ben-Efraim S, Spirer Z (1999) Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol 21(11):759–775
Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ (2004) Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15(6):2707–2719
Germain L, Jean A, Auger FA, Garrel DR (1994) Human wound healing fibroblasts have greater contractile properties than dermal fibroblasts. J Surg Res 57(2):268–273
Moulin VJ, Mayrand D, Messier H, Martinez MC, Lopez-Valle CA, Genest H (2010) Shedding of microparticles by myofibroblasts as mediator of cellular cross-talk during normal wound healing. J Cell Physiol 225(3):734–740
Corriveau MP, Boufaied I, Lessard J, Chabaud S, Senecal JL, Grodzicky T, Chartier S, Raymond Y, Moulin VJ (2009) The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: reconstitution of skin fibrosis development using a tissue engineering approach. J Pathol 217(4):534–542
Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L (1998) Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem 46(11):1291–1301
Auger FA, Lopez Valle CA, Guignard R, Tremblay N, Noel B, Goulet F, Germain L (1995) Skin equivalent produced with human collagen. In Vitro Cell Dev Biol Anim 31(6):432–439
Wiznerowicz M, Trono D (2003) Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 77(16):8957–8961
Dvorak HF (2005) Angiogenesis: update 2005. J Thromb Haemost 3(8):1835–1842
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9(2):267–285
Raffetto JD, Khalil RA (2008) Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75(2):346–359
Eming SA, Brachvogel B, Odorisio T, Koch M (2007) Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem 42(3):115–170
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601
Steinbrech DS, Longaker MT, Mehrara BJ, Saadeh PB, Chin GS, Gerrets RP, Chau DC, Rowe NM, Gittes GK (1999) Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J Surg Res 84(2):127–133
O’Cearbhaill ED, Murphy M, Barry F, McHugh PE, Barron V (2010) Behavior of human mesenchymal stem cells in fibrin-based vascular tissue engineering constructs. Ann Biomed Eng 38(3):649–657
Pelham RJ Jr, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94(25):13661–13665
Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB (2001) NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222(2):218–227
Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM (2006) Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290(2):H547–H559
Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60(1):107–114
Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K (2006) The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63(5):601–615
Ho QT, Kuo CJ (2007) Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 39(7–8):1349–1357
Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA (1998) Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 152(6):1445–1452
Murakami M, Simons M (2008) Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol 15(3):215–220
Davis GE, Saunders WB (2006) Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11(1):44–56
Noel A, Maillard C, Rocks N, Jost M, Chabottaux V, Sounni NE, Maquoi E, Cataldo D, Foidart JM (2004) Membrane associated proteases and their inhibitors in tumour angiogenesis. J Clin Pathol 57(6):577–584
Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477(1–2):267–283
Stetler-Stevenson WG (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1(27):re6
McKaig BC, McWilliams D, Watson SA, Mahida YR (2003) Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol 162(4):1355–1360
Tomlinson J, Barsky SH, Nelson S, Singer S, Pezeshki B, Lee MC, Eilber F, Nguyen M (1999) Different patterns of angiogenesis in sarcomas and carcinomas. Clin Cancer Res 5(11):3516–3522
Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP (2004) TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res 301(2):158–167
Varani J, Perone P, Warner RL, Dame MK, Kang S, Fisher GJ, Voorhees JJ (2008) Vascular tube formation on matrix metalloproteinase-1-damaged collagen. Br J Cancer 98(10):1646–1652
Aplin AC, Zhu WH, Fogel E, Nicosia RF (2009) Vascular regression and survival are differentially regulated by MT1-MMP and TIMPs in the aortic ring model of angiogenesis. Am J Physiol Cell Physiol 297(2):471–480
Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE (2006) Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175(1):179–191
Acknowledgments
The authors would like to acknowledge the Canadian Institutes of Health Research, Fondation du CHA-Hôpital Enfant-Jesus/Saint-Sacrement and Thecell network. The authors also want to thank Benoît Cordier and Samuel Blanchette for technical assistance. V. M. was the recipient of a scholarship from Fonds de la Recherche en Santé du Québec.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayrand, D., Laforce-Lavoie, A., Larochelle, S. et al. Angiogenic properties of myofibroblasts isolated from normal human skin wounds. Angiogenesis 15, 199–212 (2012). https://doi.org/10.1007/s10456-012-9253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9253-5