Abstract
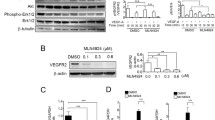
Vascular Endothelial Growth Factor-A (VEGF-A) is a key molecule in normal and tumor angiogenesis. This study addresses the role of c-ABL as a novel downstream target of VEGF-A in primary Human Umbilical Vein Endothelial Cells (HUVEC). On the basis of immunoprecipitation experiments, in vitro kinase assay and RNA interference, we demonstrate that VEGF-A induces the c-ABL kinase activity through the VEGF Receptor-2/Phosphatidylinositol-3-Kinase pathway. By treating HUVEC with the specific tyrosine kinase inhibitor STI571 and over-expressing a dominant negative c-ABL mutant, we show that the VEGF-A-activated c-ABL reduces the amplitude of Mitogen-Activated Protein Kinases (ERK1/2, JNKs and p38) activation in a dose-dependent manner by a negative feedback mechanism. By analysis of the adaptor proteins NCK1 and GRB2 mutants we further show that the negative loop on p38 is mediated by c-ABL phosphorylation at tyrosine 105 of the adaptor protein NCK1, while the phosphorylation at tyrosine 209 of GRB2 down-modulates ERK1/2 and JNKs signaling. These findings suggest that c-ABL function is to establish a correct and tightly controlled response of endothelial cells to VEGF-A during the angiogenic process.






Similar content being viewed by others
References
Ferrara N (2000) VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 11:617–624
Ferrara N (2002) Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 29:10–14
Rousseau S, Houle F, Huot J (2000) Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med 10:321–327
Salameh A, Galvagni F, Anselmi F, De Clemente C, Orlandini M, Oliviero S (2010) Growth factor stimulation induces cell survival by c-Jun·ATF2-dependent activation of Bcl-XL. J Biol Chem 285:23096–23104
Kearney JB, Ambler CA, Monaco K-A, Johnson N, Rapoport RG, Bautch VL (2002) Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 99:2397–2407
Dougher M, Terman BI (1999) Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 18:1619–1627
Takahashi T, Yamaguchi S, Chida K, Shibuya M (2001) A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J 20:2768–2778
Lamalice L, Houle F, Huot J (2006) Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem 281:34009–34020
Takahashi T, Ueno H, Shibuya M (1999) VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18:2221–2230
Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ, Royal I (2007) The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J Biol Chem 282:7758–7769
Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, Claesson-Welsh L, Welsh M (2004) The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem 279:22267–22275
Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I (2001) Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J 360:255–264
Galvagni F, Pennacchini S, Salameh A, Rocchigiani M, Neri F, Orlandini M, Petraglia F, Gotta S, Sardone GL, Matteucci G, Terstappen GC, Oliviero S (2010) Endothelial cell adhesion to the extracellular matrix induces c-Src-dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ Res 106:1839–1848
Melo JV (1996) The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 88:2375–2384
Hantschel O, Superti-Furga G (2004) Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Natl Rev Mol Cell Biol 5:33–44
Levy D, Adamovich Y, Reuven N, Shaul Y (2008) Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell 29:350–361
Barilá D, Rufini A, Condo I, Ventura N, Dorey K, Superti-Furga G, Testi R (2003) Caspase-dependent cleavage of c-Abl contributes to apoptosis. Mol Cell Biol 23:2790–2799
Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY (1996) Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci USA 93:15174–15179
Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D (2000) Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem 275:17237–17240
Li S, Couvillon AD, Brasher BB, Van Etten RA (2001) Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling. EMBO J 20:6793–6804
Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM (1999) c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 13:2400–2411
Srinivasan D, Kaetzel DM, Plattner R (2009) Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal 21:1143–1150
Frasca F, Pandini G, Malaguarnera R, Mandarino A, Messina RL, Sciacca L, Belfiore A, Vigneri R (2007) Role of c-Abl in directing metabolic versus mitogenic effects in insulin receptor signaling. J Biol Chem 282:26077–26088
Yan W, Bentley B, Shao R (2008) Distinct angiogenic mediators are required for basic fibroblast growth factor- and vascular endothelial growth factor-induced angiogenesis: the role of cytoplasmic tyrosine kinase c-Abl in tumor angiogenesis. Mol Biol Cell 19:2278–2288
Galvagni F, Anselmi F, Salameh A, Orlandini M, Rocchigiani M, Oliviero S (2007) Vascular endothelial growth factor receptor-3 activity is modulated by its association with caveolin-1 on endothelial membrane. Biochemistry 46:3998–4005
VandenDriessche T, Naldini L, Collen D, Chuah MK (2002) Oncoretroviral and lentiviral vector-mediated gene therapy. Methods Enzymol 346:573–589
Hashimoto Y, Katayama H, Kiyokawa E, Ota S, Kurata T, Gotoh N, Otsuka N, Shibata M, Matsuda M (1998) Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor. J Biol Chem 273:17186–17191
Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Grignani F, Lanfrancone L, Peschle C, Nolan GP, Pelicci PG (1998) High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res 58:14–19
Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S (2005) Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood 106:3423–3431
Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S (2004) Identification of Flk-1-target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood 103:4536–4544
Ory DS, Neugeboren BA, Mulligan RC (1996) A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA 93:11400–11406
Dougher-Vermazen M, Hulmes JD, Bohlen P, Terman BI (1994) Biological activity and phosphorylation sites of the bacterially expressed cytosolic domain of the KDR VEGF-receptor. Biochem Biophys Res Commun 205:728–738
Kou R, SenBanerjee S, Jain MK, Michel T (2005) Differential regulation of vascular endothelial growth factor receptors (VEGFR) revealed by RNA interference: interactions of VEGFR-1 and VEGFR-2 in endothelial cell signaling. Biochemistry 44:15064–15073
Wilkes MC, Leof EB (2006) Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem 281:27846–27854
Gerber H-P, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for FLK-1/KDR activation. J Biol Chem 273:30336–30343
Pedram A, Razandi M, Levin ER (1998) Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem 273:26722–26728
Stoletov KV, Ratcliffe KE, Spring SC, Terman BI (2001) NCK and PAK participate in the signaling pathway by which vascular endothelial growth factor stimulates the assembly of focal adhesions. J Biol Chem 276:22748–22755
Duval M, Bédard-Goulet S, Delisle C, Gratton J-P (2003) Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. J Biol Chem 278:20091–20097
Cujec TP, Medeiros PF, Hammond P, Rise C, Kreider BL (2002) Selection of v-Abl tyrosine kinase substrate sequences from randomized peptide and cellular proteomic libraries using mRNA display. Chem Biol 9:253–264
Songyang Z, Carraway KL, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, Schlessinger J, Hubbard SR, Smith DP, Eng C, Lorenzo MJ, Ponder BAJ, Mayer BJ, Cantley LC (1995) Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373:536–539
Barilá D, Superti-Furga G (1998) An intramolecular SH3-domain interaction regulates c-Abl activity. Nat Genet 18:280–282
Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM (2004) Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 113:516–527
Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT (2002) Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16:2684–2698
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887
O’Neill AJ, Cotter TG, Russell JM, Gaffney EF (1997) Abl expression in human fetal and adult tissues, tumours, and tumour microvessels. J Pathol 183:325–329
Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M (2005) Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev 16:159–178
Hake MJ, Choowongkomon K, Kostenko O, Carlin CR, Sönnichsen FD (2008) Specificity determinants of a novel Nck interaction with the Juxtamembrane domain of the epidermal growth factor receptor. Biochemistry 47:3096–3108
Gong C, Stoletov KV, Terman BI (2004) VEGF treatment induces signaling pathways that regulate both actin polymerization and depolymerization. Angiogenesis 7:313–322
Rohatgi R, Nollau P, Ho H-YH, Kirschner MW, Mayer BJ (2001) Nck and Phosphatidylinositol 4,5-Bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem 276:26448–26452
Kroll J, Waltenberger J (1997) The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem 272:32521–32527
Lewitzky M, Kardinal C, Gehring NH, Schmidt EK, Konkol B, Eulitz M, Birchmeier W, Schaeper U, Feller SM (2001) The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene 20:1052–1062
Acknowledgments
We thank Beatrice Grandi for technical support, Dr. Daniela Barilà (University of Rome “Tor Vergata”) for c-ABL constructs, Dr. Michiyuki Matsuda (Department of Pathology and Biology of Diseases, Kyoto University, Japan) for CRKII constructs and Dr. Shaoguang Li (The Jackson Laboratory, Bar Harbor, ME, USA) for GRB2 constructs. This study was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC), Ministero Italiano dell’Istruzione, dell’Università e della Ricerca (MIUR), Fondazione Monte dei Paschi di Siena (MPS) and Istituto Toscano Tumori (ITT).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anselmi, F., Orlandini, M., Rocchigiani, M. et al. c-ABL modulates MAP kinases activation downstream of VEGFR-2 signaling by direct phosphorylation of the adaptor proteins GRB2 and NCK1. Angiogenesis 15, 187–197 (2012). https://doi.org/10.1007/s10456-012-9252-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9252-6