Abstract
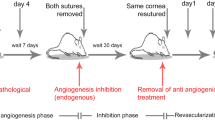
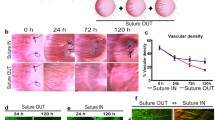
In this study, we introduce a technique for repeated, microscopic observation of single regressing capillaries in vivo in inflamed murine corneas. Natural capillary regression was initiated by removal of inflammatory stimulus during an active pro-angiogenic phase, while the additional impact of anti-angiogenic treatment with triamcinolone or bevazicumab was investigated. Capillaries regressed naturally within 1 week and treatments did not further enhance the natural regression. Morphologically, early-phase regression was characterized by significant lumen narrowing and a significant reduction in CD11b+ myeloid cell infiltration of the extracellular matrix. By 1 week, vascular remodeling occurred concomitant with CD11b+CD68+KiM2R+ mature macrophage localization on capillary walls. Empty conduits without blood flow, positive for collagen IV and devoid of vascular endothelium and pericytes, were apparent in vivo and by 3 weeks were more numerous than perfused capillaries. By 3 weeks, macrophages aggregated around remaining perfused capillaries and were observed in apposition with degrading capillary segments. Abrupt termination of capillary sprouting in our regression model further revealed vascular endothelial abandonment of sprout tips and perfused capillary loop formation within a single angiogenic sprout, possibly as an intussusceptive response to cessation of the stimulus. Finally, we observed lumen constriction and macrophage localization on capillary walls in vivo in a clinical case of corneal capillary regression that paralleled findings in our murine model.













Similar content being viewed by others
References
Lang RA, Bishop JM (1993) Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74:453–462
Meeson A, Palmer M, Calfon M, Lang R (1996) A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development 122:3929–3938
Clark RA (1993) Biology of dermal wound repair. Dermatol Clin 11:647–666
Davis GE, Saunders WB (2006) Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Invest Dermatol 11:44–56. doi:10.1038/sj.jidsymp.5650008
Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA (2007) In vivo immunostaining demonstrates macrophages associated with growing and regressing vessels. Invest Ophthalmol Vis Sci 48:4335–4341. doi:10.1167/iovs.07-0113
Lobov IB, Sujata R, Carrol TJ et al (2005) WNT7b mediates macrophage-induced programmed cell death in pattering of the vasculature. Nature 15:417–421. doi:10.1038/nature03928
Davies MH, Stempel AJ, Powers MR (2008) MCP-1deficiency delays regression of pathologic retinal neovascularization in a model of ischemic retinopathy. Invest Ophthalmol Vis Sci 49:4195–4202. doi:10.1167/iovs.07-1491
Modlich U, Kaup FJ, Augustin HG (1996) Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest 74:771–780
Hotz B, Buhr HJ, Hotz HG (2008) Intravital microscopic characterization of suramin effects in an orthotopic immunocompetent rat model of pancreatic cancer. J Gastrointest Surg 12:900–906. doi:10.1007/s11605-008-0507-x
Ausprunk DH, Falterman K, Folkman J (1978) The sequence of events in the regression of corneal capillaries. Lab Invest 3:284–294
Cursiefen C, Maruyama K, Jackson DG, Streilen W, Kruse FE (2006) Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 25:443–447. doi:10.1097/01.ico.0000183485.85636.ff
Mwaikambo BR, Sennlaub F, Ong H, Chemtob S, Hardy P (2006) Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Invest Ophthalmol Vis Sci 47:4356–4364. doi:10.1167/iovs.05-1656
Jo N, Mailos C, Ju M et al (2006) Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 168:2036–2053. doi:10.2353/ajpath.2006.050588
Brown W (2010) A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimer Dis 21:725–739. doi:10.3233/JAD-2010-100219
McKay TI, Gedeon DJ, Vickerman MB, Hylton AG, Ribita D, Olar HH, Kaiser PK, Parsons-Wingerter P (2008) Selective inhibition of angiogenesis in small blood vessels and decrease in vessel diameter throughout the vascular tree by triamcinolone acetonide. Invest Ophthalmol Vis sci 49:1184–1190. doi:10.1167/iovs.07-1329
Hoffart L, Matonti F, Conrath J, Daniel L, Ridings B, Masson G, Chavane F (2010) Inhibition of corneal neovascularization after alkali burn:comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Exp Ophthalmol 38:346–352. doi:10.1111/j.1442-9071.2010.02252.x
Kang S, Chung SK (2010) The effect of subconjuntival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea 29:192–196. doi:10.1097/ICO.0b013e3181b1c82f
Subramanian ML, Ness S, Abedi G et al (2009) Bevacizumab versus ranibizumab for age-related macular degeneration: early results of a prospective double-masked, randomized clinical trial. Am J Ophthalmol 148:875–882. doi:10.1016/j.ajo.2009.07.009
Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, Balacco Gabrieli C (2009) Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab—a randomized controlled trial. Am J Ophthalmol 149:458–464. doi:10.1016/j.ajo.2009.10.010
Abdelhakim MASE, Macky TA, Mansour KAG, Mortada HA (2011) Bevacizumab (Avastin) as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Ophthalmic Res 45:23–30. doi:10.1159/000314721
Zaki AA, Farid SF (2010) Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol 88:868–871. doi:10.1111/j.1755-3768.2009.0158.x
Dastjerdi MH, Al-Arfaj KM, Nallasamy N et al (2009) Topical bevacizumab in the treatment of corneal neovascularization. Arch Ophthalmol 127:381–389. doi:0.1001/archophthalmol.2009.18
Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A (2008) Subconjunctival bevacizumab injection for corneal neovascularization. Cornea 27:142–147. doi:10.1097/ICO.0b013e318159019f
Koening Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C (2009) Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin®) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 247:1375–1382. doi:10.1007/s00417-009-1099-1
Bock F, Onderka J, Dietrich T et al (2007) Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthlamol Vis Sci 48:2545–2552. doi:11.1167/iovs.06-0570
Han YS, Lee JE, Jung JW, Lee JS (2009) Inhibitory effects of bevacizumab on angiogenesis and corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 247:541–548. doi:10.1007/s00417-008-0976-3
Oh JY, Kim MK, Shin MS, Lee HJ, Lee JH, Wee WR (2009) The anti-inflammatory effect of subconjunctival bevacizumab on chemically burned rat corneas. Curr Eye Res 34:85–91. doi:10.1080/02713680802607740
Dastjerdi M, Saban D, Okanobo A et al (2010) Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci 51:2411–2417. doi:10.1167/iovs.09-3745
Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme JavaloyJ, Alió J (2010) Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and sunitinib (ant-VEGF and anti-PDGF) in an animal model. Am J Ophthalmol 150:519–528. doi:10.1016/j.ajo.2010.04.024
Chen WL, Lin CT, Lin NT et al (2009) Subconjunctival injection of bevacizumab (Avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci 50:1659–1665. doi:10.1167/iovs.08-1997
Drativman-Storobinsky O, Lubin B, Hasanreisoglu M, Goldenberg-Cohen N (2009) Effect of subconjunctival and intra-ocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis 15:2326–2338
Avisar I, Weinberger D, Kremer I (2010) Effect of subconjunctival, intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res 35:108–115. doi:10.3109/02713680903429007
Bourghardt Peebo B, Fagerholm P, Traneus-Röckert C, Lagali N (2011) Time-lapse in vivo imaging of corneal angiogenesis: the role of inflammatory cells in capillary sprouting. Invest Ophthalmol Vis Sci 52:3060–3068. doi:10.1167/iovs.10-6101
Peebo BB, Fagerholm P, Traneus-Röckert C, Lagali N (2010) Cellular-level characterization of lymph vessels in live, un-labeled corneas by in vivo confocal microscopy. Invest Ophthalmol Vis Sci 51:830–835. doi:10.1167/iovs.09-4407
Yu L, Wu X, Cheng Z et al (2008) Interaction between bevacizumab and murine VEGF-A: a reassessment. Invets Ophthalmol Vis Sci 49:522–527. doi:10.1167/iovs.07-1175
Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukocyte Biol. 55:410–422
Capoccia B, Shepherd RM, Link DC (2006) G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood 108:2438–2444. doi:10.1182/blood-2006-04-013755
Shojaei F, Wu X, Malik AK et al (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b + Gr1 + myeloid cells. Nature Biotech 8:911–920. doi:10.1038/nbt1323
Hillen F, Griffioen AW (2007) Tumor vascularization: sprouting angiogenesis and beyond. Cancer Metastais Review 26:489–502. doi:10.1007/s10555-007-9094-7
Makanaya AN, Hlushchuk R, Djonov VG (2008) Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning and remodeling. Angiogenesis 12:113–123. doi:10.1007/s10456-009-9129-5
Hlushchuk R, Riesterer O, Baum O, Wood J, Gruber G, Pruschy M, Djonov V (2008) Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am J Path 173:1173–1185. doi:10.2353/ajpath.2008.071131
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peebo, B.B., Fagerholm, P., Traneus-Röckert, C. et al. Cellular level characterization of capillary regression in inflammatory angiogenesis using an in vivo corneal model. Angiogenesis 14, 393–405 (2011). https://doi.org/10.1007/s10456-011-9223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-011-9223-3