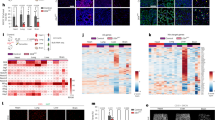
Abstract
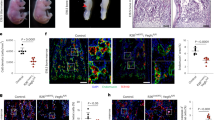
Recently, we have shown that Wnt2 is an autocrine growth and differentiation factor for hepatic sinusoidal endothelial cells. As Wnt signaling has become increasingly important in vascular development and cancer, we analyzed Wnt signaling in non-sinusoidal endothelial cells of different vascular origin (HUVEC, HUAEC, HMVEC-LLy). Upon screening the multiple components of the Wnt pathway, we demonstrated lack of Wnt2 expression, but presence of Frizzled-4, one of its receptors, in cultured non-sinusoidal endothelial cells. Treatment of these cells by exogenous Wnt2 induced endothelial proliferation and sprouting angiogenesis in vitro. Upon analysis of Wnt2 tissue expression as a basis for paracrine Wnt2 effects on non-sinusoidal endothelial cells in vivo, Wnt2 was found to be expressed in densely vascularized murine malignant tumors and in wound healing tissues in close proximity to CD31+ endothelial cells. By gene profiling, stanniocalcin-1 (STC1), a known regulator of angiogenesis, was identified as a target gene of Wnt2 signaling in HUVEC down-regulated by Wnt2 treatment. Tumor-conditioned media counter-acted Wnt2 and up-regulated STC1 expression in HUVEC. In conclusion, we provide evidence that Wnt2 acts as an angiogenic factor for non-sinusoidal endothelium in vitro and in vivo whose target genes undergo complex regulation by the tissue microenvironment.







Similar content being viewed by others
References
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Goodwin AM, D’Amore PA (2002) Wnt signaling in the vasculature. Angiogenesis 5:1–9. doi:10.1023/A:1021563510866
You L, He B, Xu Z et al (2004) An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res 64:5385–5389. doi:10.1158/0008-5472.CAN-04-1227
Mazieres J, You L, He B et al (2005) Wnt2 as a new therapeutic target in malignant pleural mesothelioma. Int J Cancer 117:326–332. doi:10.1002/ijc.21160
Wright M, Aikawa M, Szeto W, Papkoff J (1999) Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun 263:384–388. doi:10.1006/bbrc.1999.1344
Cheng CW, Smith SK, Charnock-Jones DS (2003) Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res 291:415–425. doi:10.1016/j.yexcr.2003.07.006
Ishikawa T, Tamai Y, Zorn AM et al (2001) Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128:25–33
Hsieh M, Boerboom D, Shimada M et al (2005) Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 73:1135–1146. doi:10.1095/biolreprod.105.042739
Nikolova T, Wu M, Brumbarov K et al (2007) WNT-conditioned media differentially affect the proliferation and differentiation of cord blood-derived CD133+ cells in vitro. Differentiation 75:100–111. doi:10.1111/j.1432-0436.2006.00119.x
Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J (2005) Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 8:43–51. doi:10.1007/s10456-005-5612-9
Zerlin M, Julius MA, Kitajewski J (2008) Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11:63–69. doi:10.1007/s10456-008-9095-3
Biswas P, Canosa S, Schoenfeld J et al (2003) PECAM-1 promotes beta-catenin accumulation and stimulates endothelial cell proliferation. Biochem Biophys Res Commun 303:212–218. doi:10.1016/S0006-291X(03)00313-9
Wu WB, Peng HC, Huang TF (2003) Disintegrin causes proteolysis of beta-catenin and apoptosis of endothelial cells. Involvement of cell–cell and cell–ECM interactions in regulating cell viability. Exp Cell Res 286:115–127. doi:10.1016/S0014-4827(03)00105-8
Klein D, Demory A, Peyre F et al (2008) Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology 47:1018–1031. doi:10.1002/hep.22084
Goodwin AM, Kitajewski J, D’Amore PA (2007) Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 25:25–32. doi:10.1080/08977190701272933
Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ (1996) Targeted disruption of the Wnt2 gene results in placentation defects. Development 122:3343–3353
Giovarelli M, Musiani P, Modesti A et al (1995) Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol 155:3112–3123
Falkowski M, Schledzewski K, Hansen B, Goerdt S (2003) Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem Cell Biol 120:361–369. doi:10.1007/s00418-003-0585-5
Korff T, Augustin HG (1998) Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 143:1341–1352. doi:10.1083/jcb.143.5.1341
Zheng C, Li L, Haak M et al (2006) Gene expression profiling of CD34+ cells identifies a molecular signature of chronic myeloid leukemia blast crisis. Leukemia 20:1028–1034. doi:10.1038/sj.leu.2404227
Chu TM, Weir B, Wolfinger R (2002) A systematic statistical linear modeling approach to oligonucleotide array experiments. Math Biosci 176:35–51. doi:10.1016/S0025-5564(01)00107-9
Goodwin AM, Sullivan KM, D’Amore PA (2006) Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn 235:3110–3120. doi:10.1002/dvdy.20939
Xu Q, Wang Y, Dabdoub A et al (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895. doi:10.1016/S0092-8674(04)00216-8
Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14:59–88. doi:10.1146/annurev.cellbio.14.1.59
Nusse R (2005) Wnt signaling in disease and in development. Cell Res 15:28–32. doi:10.1038/sj.cr.7290260
Watanabe O, Imamura H, Shimizu T et al (2004) Expression of twist and Wnt in human breast cancer. Anticancer Res 24:3851–3856
Cheng XX, Wang ZC, Chen XY et al (2005) Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett 223:339–347. doi:10.1016/j.canlet.2004.11.013
You L, He B, Xu Z et al (2004) Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene 23:6170–6174. doi:10.1038/sj.onc.1207844
Varghese R, Gagliardi AD, Bialek PE et al (2002) Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 143:868–876. doi:10.1210/en.143.3.868
Holmes DI, Zachary IC (2008) Vascular endothelial growth factor regulates stanniocalcin-1 expression via neuropilin-1-dependent regulation of KDR and synergism with fibroblast growth factor-2. Cell Signal 20:569–579. doi:10.1016/j.cellsig.2007.11.009
Yeung HY, Lai KP, Chan HY et al (2005) Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146:4951–4960. doi:10.1210/en.2005-0365
Zlot C, Ingle G, Hongo J et al (2003) Stanniocalcin 1 is an autocrine modulator of endothelial angiogenic responses to hepatocyte growth factor. J Biol Chem 278:47654–47659. doi:10.1074/jbc.M301353200
Gerritsen ME, Tomlinson JE, Zlot C et al (2003) Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol 140:595–610. doi:10.1038/sj.bjp.0705494
Apte U, Zeng G, Muller P et al (2006) Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology 44:992–1002. doi:10.1002/hep.21317
David M, Yeramian A, Dunach M et al (2008) Signaling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential β-catenin phosphorylation. J Cell Sci 121:2718–2730. doi:10.1242/jcs.029660
Wascher RA, Huynh KT, Giuliano AE et al (2003) Stanniocalcin-1: a novel molecular blood and bone marrow marker for human breast cancer. Clin Cancer Res 9:1427–1435
Acknowledgments
Grant support: The work was supported by the Deutsche Forschungsgemeinschaft [SFB TR 23, (S.G. and B.A.); European Graduate College GRK 880/2 (S.G.)] and by the Tumor Centre Heidelberg/Mannheim (S.G. and B.A.). The authors state that there are no personal or institutional conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klein, D., Demory, A., Peyre, F. et al. Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis 12, 251–265 (2009). https://doi.org/10.1007/s10456-009-9145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-009-9145-5