Abstract
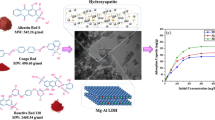
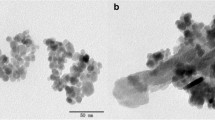
A novel magnetic hydroxyapatite-supported nickel oxide nanocomposite (NiO–HAP@γ-Fe2O3) was successfully prepared using a combination of co-precipitation and wet impregnation methods and was applied to the adsorption of methylene blue from aqueous solution. The presence of HAP, γ-Fe2O3, NiO and all elements in NiO–HAP@γ-Fe2O3 was confirmed by XRD, SEM–EDX and ICP-AES. The structure of the resulting nanocomposite was shown by TEM and SEM–EDX to be rod-shaped, measuring 55.8 ± 16.5 nm in length and 27.1 ± 6.2 nm in width, and on the surface of which was uniformly interspersed with NiO nanoparticles (about 21.4 nm average crystallite size) and γ-Fe2O3 nanoparticles (6.7 ± 2.6 nm in diameter). The novel NiO–HAP@γ-Fe2O3 exhibited a high adsorption rate during the first 20 min and reached an equilibrium within 3 h. The adsorption capacity of NiO–HAP@γ-Fe2O3 was significantly higher than that of its precursors (7.20 mg g−1 vs 0.79–1.31 mg g−1). The superior adsorption performance of the novel nanocomposite, which occurred despite its relatively low surface area, is likely attributable to the synergistic mechanisms facilitated by the presence of mixed metal oxides (NiO and γ-Fe2O3) on the adsorbent as well as by the Lewis acidity and basicity of the components of the adsorbent and the adsorbate. The adsorption kinetics and isotherms were well-fitted by the pseudo-second-order kinetic model and the Langmuir isotherm model, respectively.










Similar content being viewed by others
References
Adhikari, S., Mandal, S., Sarkar, D., Kim, D.-H., Madras, G.: Kinetics and mechanism of dye adsorption on WO3 nanoparticles. Appl. Surf. Sci. 420, 472–482 (2017)
Aghabeygi, S., Kojoori, R.K., Azad, H.V.: Sonosynthesis, characterization and photocatalytic degradation property of nano ZnO/zeolite A. Iran. J. Catal. 6, 275–279 (2016)
Ajoudanian, N., Nezamzadeh-Ejhieh, A.: Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater. Sci. Semicond. Process. 36, 162–169 (2015)
Alkan, M., Demirbaş, Ö, Doğan, M.: Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 101(3), 388–396 (2007)
Anari-Anaraki, M., Nezamzadeh-Ejhieh, A.: Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interface Sci. 440, 272–281 (2015)
Ayad, M., El-Hefnawy, G., Zaghlol, S.: Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem. Eng. J. 217, 460–465 (2013)
Bhattacharyya, K., Sharma, A.: Kinetics and thermodynamics of methylene blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes Pigm. 65(1), 51–59 (2005)
Borandegi, M., Nezamzadeh-Ejhieh, A.: Enhanced removal efficiency of clinoptilolite nano-particles toward Co(II) from aqueous solution by modification with glutamic acid. Colloids Surf. A. 479, 35–45 (2015)
Buthiyappan, A., Aziz, A., Wan Daud, A.R.: W.M.A.: Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 32(1), 1–47 (2016)
Chowdhury, A.-N., Rahim, A., Ferdosi, Y.J., Azam, M.S., Hossain, M.M.: Cobalt–nickel mixed oxide surface: a promising adsorbent for the removal of PR dye from water. Appl. Surf. Sci. 256(12), 3718–3724 (2010)
Eftekhari, S., Habibi-Yangjeh, A., Sohrabnezhad, S.: Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: thermodynamic and kinetic studies. J. Hazard. Mater. 178(1–3), 349–355 (2010)
El-Sayes, M.A.: Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 34, 257–264 (2001)
Franco, D.S.P., Piccin, J.S., Lima, E.C., Dotto, G.L.: Interpretations about methylene blue adsorption by surface modified chitin using the statistical physics treatment. Adsorption. 21(8), 557–564 (2015)
Gnida, A., Wiszniowski, J., Felis, E., Sikora, J., Surmacz-Górska, J., Miksch, K.: The effect of temperature on the efficiency of industrial wastewater nitrification and its (geno)toxicity. Arch. Environ. Prot. 42(1), 27–34 (2016)
He, C., Hu, X.: Functionalized ordered mesoporous carbon for the adsorption of reactive dyes. Adsorption. 18(5–6), 337–348 (2012)
He, H.B., Li, B., Dong, J.P., Lei, Y.Y., Wang, T.L., Yu, Q.W., Feng, Y.Q., Sun, Y.B.: Mesostructured nanomagnetic polyhedral oligomeric silsesquioxanes (POSS) incorporated with dithiol organic anchors for multiple pollutants capturing in wastewater. ACS Appl. Mater. Interfaces. 5(16), 8058–8066 (2013)
Heidari-Chaleshtori, M., Nezamzadeh-Ejhieh, A.: Clinoptilolite nano-particles modified with aspartic acid for removal of Cu(ii) from aqueous solutions: isotherms and kinetic aspects. New J. Chem. 39(12), 9396–9406 (2015)
Jang, M., Chen, W., Cannon, F.S.: Preloading hydrous ferric oxide into granular activated carbon for Aarsenic removal. Environ. Sci. Technol. 42, 3369–3374 (2008)
Kandula, S., Jeevanandam, P.: Synthesis of silica@Ni-Co mixed metal oxide core-shell nanorattles and their potential use as effective adsorbents for waste water treatment. Eur. J. Inorg. Chem. 2015(25), 4260–4274 (2015)
Karimi-Shamsabadi, M., Nezamzadeh-Ejhieh, A.: Comparative study on the increased photoactivity of coupled and supported manganese-silver oxides onto a natural zeolite nano-particles. J. Mol. Catal. A. 418–419, 103–114 (2016)
Khosravi, I., Eftekhar, M.: Na0.5Li0.5CoO2 nanopowders: facile synthesis, characterization and their application for the removal of methylene blue dye from aqueous solution. Adv. Powder Technol. 25(6), 1721–1727 (2014)
Kong, D., Zheng, X., Tao, Y., Lv, W., Gao, Y., Zhi, L., Yang, Q.-H.: Porous graphene oxide-based carbon artefact with high capacity for methylene blue adsorption. Adsorption. 22(8), 1043–1050 (2016)
Konicki, W., Sibera, D., Mijowska, E., Lendzion-Bielun, Z., Narkiewicz, U.: Equilibrium and kinetic studies on acid dye Acid Red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J. Colloid Interface Sci. 398, 152–160 (2013)
Kumar, K.Y., Muralidhara, H.B., Nayaka, Y.A., Balasubramanyam, J., Hanumanthappa, H.: Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 246, 125–136 (2013)
Largitte, L., Pasquier, R.: A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 109, 495–504 (2016)
Lei, C., Pi, M., Jiang, C., Cheng, B., Yu, J.: Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 490, 242–251 (2017)
Li, F., Wu, X., Ma, S., Xu, Z., Liu, W., Liu, F.: Adsorption and desorption mechanisms of methylene blue removal with iron-oxide coated porous ceramic filter. J. Water Resour. Prot. 1, 1–57 (2009)
Li, L.H., Xiao, J., Liu, P., Yang, G.W.: Super adsorption capability from amorphousization of metal oxide nanoparticles for dye removal. Sci. Rep. 5, 9028 (2015)
Lin, K.-S., Cheng, H.-W., Chen, W.-R., Wu, C.-F.: Synthesis, characterization, and adsorption kinetics of titania nanotubes for basic dye wastewater treatment. Adsorption. 16(1–2), 47–56 (2010)
Lorenc-Grabowska, E., Gryglewicz, G.: Adsorption of lignite-derived humic acids on coal-based mesoporous activated carbons. J. Colloid Interface Sci. 284(2), 416–423 (2005)
Ma, J., Yu, F., Zhou, L., Jin, L., Yang, M., Luan, J., Tang, Y., Fan, H., Yuan, Z., Chen, J.: Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces. 4(11), 5749–5760 (2012)
Ma, D., Zhu, B., Cao, B., Wang, J., Zhang, J.: Fabrication of the novel hydrogel based on waste corn stalk for removal of methylene blue dye from aqueous solution. Appl. Surf. Sci. 422, 944–952 (2017)
Mandal, S., Natarajan, S.: Adsorption and catalytic degradation of organic dyes in water using ZnO/ZnxFe3–xO4 mixed oxides. J. Environ. Chem. Eng. 3(2), 1185–1193 (2015)
Miessler, G.L., Fischer, P.J., Tarr, D.A.: Inorganic Chemistry, 5 edn. Pearson Education, Essex (2014)
Muthukumaran, C., Sivakumar, V.M., Thirumarimurugan, M.: Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan Inst. Chem. Eng. 63, 354–362 (2016)
Ncibi, M.C., Mahjoub, B., Seffen, M.: Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres. J. Hazard. Mater. 139(2), 280–285 (2007)
Nezamzadeh-Ejhieh, A., Zabihi-Mobarakeh, H.: Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 20(4), 1421–1431 (2014)
Rong, X., Qiu, F., Zhang, C., Fu, L., Wang, Y., Yang, D.: Adsorption–photodegradation synergetic removal of methylene blue from aqueous solution by NiO/graphene oxide nanocomposite. Powder Technol. 275, 322–328 (2015)
Sajjadifar, S., Abbasi, Z., Rezaee Nezhad, E., Moghaddam, M.R., Karimian, S., Miri, S.: Ni2+ supported on hydroxyapatite-core-shell γ-Fe2O3 nanoparticles: a novel, highly efficient and reusable lewis acid catalyst for the regioselective azidolysis of epoxides in water. J. Iran. Chem. Soc. 11(2), 335–340 (2013)
Saoiabi, S., Achelhi, K., Masse, S., Saoiabi, A., Laghzizil, A., Coradin, T.: Organo-apatites for lead removal from aqueous solutions: a comparison between carboxylic acid and aminophosphonate surface modification. Colloids Surf., A. 419, 180–185 (2013)
Satheesh, R., Vignesh, K., Rajarajan, M., Suganthi, A., Sreekantan, S., Kang, M., Kwak, B.S.: Removal of congo red from water using quercetin modified α-Fe2O3 nanoparticles as effective nanoadsorbent. Mater. Chem. Phys. 180, 53–65 (2016)
Singh, S.A., Vemparala, B., Madras, G.: Adsorption kinetics of dyes and their mixtures with Co3O4–ZrO2 composites. J. Environ. Chem. Eng. 3(4), 2684–2696 (2015)
Sivashankar, R., Sathya, A.B., Vasantharaj, K., Sivasubramanian, V.: Magnetic composite an environmental super adsorbent for dye sequestration—a review. Environ. Nanotechnol. Monit. Manag. 1–2, 36–49 (2014)
Travlou, N.A., Kyzas, G.Z., Lazaridis, N.K., Deliyanni, E.A.: Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir. 29(5), 1657–1668 (2013)
Wei, W., Yang, L., Zhong, W.H., Li, S.Y., Cui, J., Wei, Z.G.: Fast removal of methylene blue from aquous solution by adsorption onto poorly crystalline hydroxyapatite nanoparticles. Dig. J. Nanomater. Biostruct. 10, 1343–1363 (2015)
Yan, B., Chen, Z., Cai, L., Chen, Z., Fu, J., Xu, Q.: Fabrication of polyaniline hydrogel: synthesis, characterization and adsorption of methylene blue. Appl. Surf. Sci. 356, 39–47 (2015)
Zhu, B., Xia, P., Ho, W., Yu, J.: Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 344, 188–195 (2015)
Acknowledgements
This work was supported by Grants for Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phasuk, A., Srisantitham, S., Tuntulani, T. et al. Facile synthesis of magnetic hydroxyapatite-supported nickel oxide nanocomposite and its dye adsorption characteristics. Adsorption 24, 157–167 (2018). https://doi.org/10.1007/s10450-017-9931-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-017-9931-0