Abstract
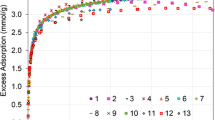
Considering the great economic and environmental interests in the capture and separation of CO2 and the wide availability of faujasites zeolites (FAU), we propose a set of parameters based on classical force fields that has good transferability among Na-FAU sieves and CO2. In addition to CO2, the parameterization strategy was tested for H2S, O2, N2 and CH4 gases. For these gases, the force field adequately predicts the adsorption isotherms at low pressure. The force field was also tested for N2 in the FAU framework with different monovalent and divalent cations, resulting in quantitative agreement for monovalent cations and qualitative agreement for divalent cations. The good tradeoff between the reliability and ease of implementation will enable rapid evaluation of the adsorption properties of gaseous mixtures of industrial relevance. The reasoning of the re-parameterization strategy is also discussed in detail.











Similar content being viewed by others
References
Akten, E.D., Siriwardane, R., Sholl, D.S.: Monte Carlo simulation of single- and binary-component adsorption of CO2, N2, and H2 in zeolite Na-4A. Energy Fuels 17, 977–983 (2003). doi:10.1021/ef0300038
Bae, T., Hudson, M.R., Mason, J.A., Queen, W.L., Dutton, J.J., Sumida, K., Micklash, K.J., Kaye, S.S., Brown, C.M., Long, J.R.: Energy Environ. Sci. 6, 128–138 (2013)
Bezus, A.G., Kiselev, A.V., Lopatkin, A.A., Du, P.Q.: Molecular statistical calculation of the thermodynamic adsorption characteristics of zeolites using the atom-atom approximation Part 1.-adsorption of methane by zeolite NaX J. Chem. Soc. Faraday Trans. 2 74, 367–379 (1978)
Calero, S., Dubbeldam, D., Krishna, R., Smit, B., Vlugt, T.J.H., Denayer, J.F.M., Martens, J.A., Maesen, T.L.M.: Understanding the role of sodium during adsorption: a force field for alkanes in sodium-exchanged faujasites. J. Am. Chem. Soc. 126, 11377–11386 (2004)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004)
Cejka, J., van Bekkum, H., Corma, A., Schueth, F.: Introduction to Zeolite Science and Practice. Studies in Surface Science and Catalysis, vol. 168, 3rd edn. Elsevier Science, Oxford (2007)
Cruz, A.J., Pires, J., Carvalho, A.P., de Carvalho, M.B.: Physical adsorption of H2S related to the conservation of works of art: the role of the pore structure at low relative pressure. Adsorption 11, 569–576 (2005)
Davies, G.M., Seaton, N.A.: The effect of the choice of pore model on the characterization of the internal structure of microporous carbons using pore size distribution. Carbon 36, 1473–1490 (1998)
Di Lella, A., Desbiens, N., Boutin, A., Demachy, I., Ungerer, P., Bellat, J.-P., Fuchs, A.H.: Molecular simulation studies of water physisorption in zeolites. Phys. Chem. Chem. Phys. 8, 5396–5406 (2006). doi:10.1039/b610621h
Dubbeldam, D., Calero, S., Vlugt, T.J.H., Krishna, R., Maesen, T.L.M., Beerdsen, E., Smit, B.: Force field parametrization through fitting on inflection points in isotherms. Phys. Rev. Lett. 93, 088302 (2004)
Fang, H., Kamakoti, P., Ravikovitch, P.I., Aronson, M., Paur, C., Sholl, D.S.: First principles derived, transferable force fields for CO2 adsorption in Na-exchanged cationic zeolites. Phys. Chem. Chem. Phys. 15, 12882–12894 (2013)
First, E.L., Faruque Hasan, M.M., Christodoulos, A.: Floudas—discovery of novel zeolites for natural gas purification through combined material screening and process optimization. AIChE J. 60, 1767–1785 (2014)
Fitch, A.N., Jobic, H., Renouprez, A.: Localization of benzene in sodium-Y-zeolite by powder neutron diffraction. J. Phys. Chem. 90, 1311–1318 (1986)
Frenkel, D., Smit, B.: Understanding molecular simulation. Academic Press, New York (2002)
García-Sánchez, A., Ania, C.O., Parra, J.B., Dubbeldam, D., Vlugt, T.J.H., Krishna, R., Calero, S.: Transferable force field for carbon dioxide adsorption in zeolites. J. Phys. Chem. C 113, 8814–8820 (2009). doi:10.1021/jp810871f
Gusev, V.Y., O’Brien, J.A., Seaton, N.A.: A self-consistent method for characterization of activated carbons using supercritical adsorption and grand canonical Monte Carlo simulations. Langmuir 13, 2815–2821 (1997)
Harris, J.G., Yung, K.H.: Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J. Phys. Chem. 99, 12021–12024 (1995)
Hyun, S.H., Danner, R.P.: Equilibrium adsorption of ethane, ethylene, isobutane, carbon dioxide and their binary mixtures. J. Chem. Eng. Data 27, 196–200 (1982)
Jayaraman, A., Yang, R.T., Cho, S.-H., Bhat, T.S.G., Choudary, V.N.: Adsorption of nitrogen, oxygen and argon on Na-CeX zeolites. Adsorption 8, 271–278 (2002)
Jaramillo, E., Chandross, M.: Adsorption of small molecules in LTA zeolites. 1. NH3, CO2, and H2O in zeolite 4A. J. Phys. Chem. B 108, 20155–20159 (2004)
Kaneco, K., Craknell, R.F., Nicholson, D.: Nitrogen adsorption in slit pores at ambient temperatures: comparison of simulation and experiment. Langmuir 10, 4606–4609 (1994)
Karge, H.G., Raskó, J.: Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied Si-Al ratios. Colloid Interface Sci. 64, 522–532 (1978)
Kim, J.N., Chue, K.T., Kim, K.I., Cho, S.H., Kim, J.D.: Nonisothermal adsorption of nitrogen-carbon dioxide mixture in a fixed-bed of zeolite-X. J. Chem. Eng. Jpn. 27, 45–51 (1994)
Kiselev, A.V., Du, P.Q.: Molecular statistical calculation of the thermodynamic adsorption characteristics of zeolites using the atom- atom approximation Part 2- adsorption of non-polar and polar inorganic molecules by zeolites of types X and Y. J. Chem. Soc. Faraday Trans. 277, 1–15 (1981)
Kitagawa, T., Tsunekawa, T., Iwayama, K.: Monte Carlo simulations on adsorptions of benzene and xylenes in sodium-Y zeolites. Microporous Mater. 7, 227–233 (1996)
Kristof, T., Liszi, J.: Effective intermolecular potential for fluid hydrogen sulfide. J. Phys. Chem. B 101, 5480–5483 (1997)
Kumar, P., Sung, C.-Y., Muraza, O., Cococcioni, M., Al Hashimi, S., McCormick, A., Tsapatsis, M.: H2S adsorption by Ag and Cu ion exchanged faujasites. Microporous Mesoporous Mater. 146, 127–133 (2011). doi:10.1016/j.micromeso.2011.05.014
Llewellyn, P.L., Maurin, G.: Gas adsorption microcalorimetry and modelling to characterize zeolites and related materials. C. R. Chim. 8, 283–302 (2005)
Liu, D., Wu, Y., Xia, Q., Li, Z., Xi, H.: Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 19, 23–37 (2013)
Liu, S., Yang, X.: Gibbs ensemble Monte Carlo simulation of supercritical CO2 adsorption on NaA and NaX zeolites. J. Chem. Phys. 124, 244705 (2006)
Lowenstein, W.: The distribution of Al in the tetrahedra of silicates and aluminates. Am. Mineral. 39, 92–96 (1954)
Lucena, S.M.P., Frutuoso, L.F.A., Silvino, P.F.G., Azevedo, D.C.S., Toso, J.P., Zgrablich, G., Cavalcante, C.L.: Molecular simulation of collection of methane isotherms in carbon material using all-atom and united atom models. Colloids Surf. A 357, 53–60 (2010)
Maurin, G., Llewellyn, P.L., Bell, R.G.: Adsorption mechanism of carbon dioxide in faujasites: grand canonical monte carlo simulations and microcalorimetry measurements. J. Phys. Chem. B 109, 16084–16091 (2005a)
Maurin, G., Llewellyn, P.L., Poyet, T., Kuchta, B.: Influence of extra-framework cations on the adsorption properties of X-faujasite systems: microcalorimetry and molecular simulations. J. Phys. Chem. B 109, 125–129 (2005b)
Simulation, GrandCanonical Monte Carlo, Measurements, Volumetric, Pillai, R.S., Sethia, G., Jasra, V.: Sorption of CO, CH4, and N2 in alkali metal ion exchanged zeolite-X. Ind. Eng. Chem. Res. 49, 5816–5825 (2010)
Plant, D.F., Simperler, A., Bell, R.G.: Adsorption of methanol on zeolites X and Y. An atomistic and quantum chemical study. J. Phys. Chem. B 110, 6170–6178 (2006)
Plévert, J., Di Renzo, F., Fajula, F.: Structure of dehydrated zeolite Li − LSX by neutron diffraction: evidence for a low-temperature orthorhombic faujasite. J. Phys. Chem. B 101, 10340–10346 (1997)
Rappé, A.K., Goddard III, W.A.: Charge equilibration for molecular dynamics simulations. J. Phys. Chem. 95, 3358–3363 (1991)
Rappé, A.K., Casewit, C.J., Cowell, K.S., Goddard III, W.A., Skiff, W.M.: UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992)
Silva, J.A.C., Schumann, K., Rodrigues, A.E.: Sorption and kinetics of CO2 and CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater. 158, 219–228 (2012)
Skarmoutsos, I., Kampanakis, L.I., Samios, J.: Investigation of the vapor–liquid equilibrium and supercritical phase of pure methane via computer simulations. J. Mol. Liq. 117, 33–41 (2005)
Su, F., Lu, C.: CO2 capture from gas stream by zeolite 13X using a dual-column temperature/vacuum swing adsorption. Energy Environ. Sci. 5, 9021–9027 (2012)
Sun, W., Lin, L.-C., Peng, X., Smit, B.: Computational screening of porous metal-organic frameworks and zeolites for the removal of SO2 and NOx from flue gases. AIChE J. 60, 2314–2323 (2014)
Sung, C.-Y., Broadbelt, Linda J., Randall, Q.: Snurr QM/MM study of the effect of local environment on dissociative adsorption in BaY zeolites. J. Phys. Chem. C 113, 15643–15651 (2009)
Sung, C.-Y., Hashimi, S.Al, McCormick, A., Tsapatsis, M., Cococcioni, M.: Density functional theory study on the adsorption of H2S and other claus process tail gas components on copper- and silver-exchanged Y zeolites. J. Phys. Chem. C 116, 3561–3575 (2012)
Tagliabue, M., Bellussi, G., Broccia, P., Carati, A., Millini, R., Pollesel, P., Rizzo, C.: Chem. Eng. J. 210, 398–403 (2012)
Takahashi, A., Yang, F.H., Yang, R.T., in: M.D. LeVan (Ed.), FOA7—Fundamentals of Adsorption 7, 578–585 (1996)
Thang, H.V., Grajciar, L., Nachtigall, P., Bludsky, O., Areán, C.O., Frydová, E., Bulánek, R.: Adsorption of CO2 in FAU zeolites: effect of zeolite composition. Catal. Today 227, 50–56 (2014)
Uytterhoeven, L., Dompas, D., Mortier, W.J.: J. Chem. Soc. Faraday Trans. 88, 2753–2760 (1992)
Vitale, G., Bull, L.M., Morris, R.E., Cheetham, A.K., Toby, B.H., Coe, C.G., Macdougall, J.E.: Combined neutron and X-ray powder diffraction study of zeolite Ca LSX and a 2H NMR study of its complex with benzene. J. Phys. Chem. B 99, 16087–16092 (1995)
Vitale, G., Mellot, C.F., Bull, L.M., Cheetham, A.K.: Neutron diffraction and computational study of zeolite NaX: influence of SIII’ cations on its complex with benzene. J. Phys. Chem. B 101, 4559 (1997)
Walton, K.S., Abney, M.B., Levan, M.D.: CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 91, 78–84 (2006)
Watanabe, K., Austin, N., Stapleton, M.R.: Investigation of the air separation properties of zeolites types A, X and Y by Monte Carlo simulations. Mol. Simul. 15, 197–221 (1995)
Wilmer, C.E., Farha, O.K., Bae, Y.-S., Hupp, J.T., Randall, Q.: Snurr—structure–property relationships of porous materials for carbon dioxide separation and capture. Energy Environ. Sci. 5, 9849–9856 (2012)
Wong-Ng, W., Kaduk, J.A., Huang, Q., Espinal, L., Li, L., Burress, J.W.: Investigation of NaY zeolite with adsorbed CO2 by neutron powder diffraction. Microporous Mesopororous Mater. 172, 95–104 (2013)
Zhu, L., Seff, K.: Reinvestigation of the crystal structure of dehydrated sodium zeolite X. J. Phys. Chem. B 103, 9512–9518 (1999)
Zhu, L., Seff, K.: Cation crowding in zeolites. Reinvestigation of the crystal structure of dehydrated potassium-exchanged zeolite X. J. Phys. Chem. B 104, 8946–8951 (2000)
Acknowledgments
This work is supported by the Brazilian research agencies, CNPq and CAPES.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gomes, V.A.M., Coelho, J.A., Peixoto, H.R. et al. Easily tunable parameterization of a force field for gas adsorption on FAU zeolites. Adsorption 21, 25–35 (2015). https://doi.org/10.1007/s10450-014-9647-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-014-9647-3