Abstract
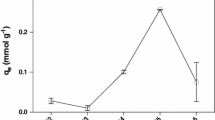
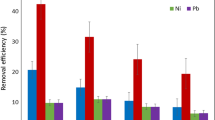
Biosorption of Cu2+ and Pb2+ by Cladop- hora fascicularis was investigated as a function of initial pH, initial heavy metal concentrations, temperature and other co-existing ions. Adsorption equilibriums were well described by Langmuir and Freundlich isotherm models. The maximum adsorption capacities were 1.61 mmol/g for Cu2+ and 0.96 mmol/g for Pb2+ at 298 K and pH 5.0. The adsorption processes were endothermic and biosorption heats calculated by the Langmuir constant b were 39.0 and 29.6 kJ/mol for Cu2+ and Pb2+, respectively. The biosorption kinetics followed the pseudo-second order model. No significant effect on the uptake of Cu2+ and Pb2+ by co-existing cations and anions was observed, except EDTA. Desorption experiments indicated that Na2EDTA was an efficient desorbent for the recovery of Cu2+ and Pb2+ from biomass. The results showed that Cladophora fascicularis was an effective and economical biosorbent material for the removal and recovery of heavy metal ions from wastewater.
Similar content being viewed by others
References
Aksu, Z., “Equilibrium and Kinetic Modelling of Cadmium (II) Biosorption by C. vulgaris in a Batch System: Effect of Temperature,” Sep. Purif. Technol., 21(3), 285–294 (2001).
Aksu, Z. and T. Kutsal, “Determination of Kinetic Parameters in the Biosorption of Copper (II) on Cladophora sp., in a Packed Bed Column Reactor,” Process Biochem., 33(1), 7–13 (1998).
Alimohamadi, M., G. Abolhamd, and A. Keshtkar, “Pb (II) and Cu (II) Biosorption on Rhizopus arrhizus Modeling Mono-and Multi-Component Systems,” Minerals Eng., 18(13–14), 1325–1330 (2005).
Andrade, A.D., M.C.E. Rollemberg, and J.A. Nóbrega, “Proton and Metal Binding Capacity of the Green Freshwater Alga Chaetophora elegans,” Process Biochem., 40(5), 1931–1936 (2005).
Chang, J.S., R. Law, and C. Chang, “Biosorption of Lead, Copper and Cadmium by Biomass of Pseudomonas aeruginosa PU21,” Wat. Res., 31(7), 1651–1658 (1997).
Diniz, V. and B. Volesky, “Biosorption of La, Eu and Yb Using Sargassum Biomass,” Wat. Res., 39(1), 239–247 (2005).
Dönmez, G.C., Z. Aksu, A. Öztürk, and T. Kutsal, “A Comparative Study on Heavy Metal Biosorption Characteristics of Some Algae,” Process Biochem., 34(9), 885–892 (1999).
Dursun, A.Y., G. Uslu, Y. Cuci, and Z. Aksu, “Bioaccumulation of Copper (II), Lead (II) and Chromium (VI) by Growing Aspergillus niger,” Process Biochem., 38(12), 1647–1651 (2003).
Figueira, M.M., B. Volesky, V.S.T. Ciminelli, and F.A. Roddick, “Biosorption of Metals in Brown Seaweed Biomass,” Wat. Res., 34(1), 196–204 (2000).
Fourest, E., C. Canal, and J.C. Roux, “Improvement of Heavy Metal Biosorption by Mycelial Dead Biomass: pH Control, and Cationic Activation,” FEMS Micobiol. Rev., 14, 332–352 (1994).
Fourest, E. and J. Roux, “Heavy Metal Biosorption by Fungal Mycelial by Product: Mechanism and Influence of pH,” Appl. Microbiol. Biotechnol., 37, 399–403 (1992).
Göksungur, Y., S. Üren, and U. Güvenç, “Biosorption of Cadmium and Lead Ions by Ethanol Treated Waste Baker’s Yeast Biomass,” Bioresource Technol., 96(1), 103–109 (2005).
Gupta, V.K., A. Rastogi, V.K. Saini, and N. Jain, “Biosorption of Copper (II) from Aqueous Solutions by Green Spirogyra Species,” J. Colloid Interf. Sci., 296(1), 59–63 (2006).
Gupta, V.K., A.K. Shrivastara, and N. Jain, “Biosorption of Chromium (VI) from Aqueous Solutions by Green Spirogyra Species,” Wat. Res., 35(7), 4079–4085 (2001).
Han, R.P., J. Zhang, W. Zou, J. Shi, and H. Liu, “Equilibrium Biosorption Isotherm for Lead Ion on Chaff,” J. Hazard. Mater., 125(1–3), 266–271 (2005).
Hansen, H.K., A. Ribeiro, and E. Mateus, “Biosorption of Arsenic (V) with Lessonia nigrescens,” Miner. Eng., 19(5), 486–490 (2006).
Hashim, M.A. and K.H. Chu, “Biosorption of Cadmium by Brown, Green and Red Seaweeds,” Chem. Eng. J., 97(2–3), 249–255 (2004).
Herrero, R., B. Cordero, P. Lodeiro, C. Rey-Castro, and M.E. Sastre de Vicente, “Interactions of Cadmium (II) and Protons with Dead Biomass of Marine Algae Fucus sp.,” Mar. Chem., 99(1–4), 106–116 (2006).
Holan, Z.R., B. Volesky, and I. Prasetyo, “Biosorption of Cadmium by Biomass of Marine Algae,” Biotech. Bioeng., 41, 819–825 (1993).
Holan, Z.R. and B. Volesky, “Biosorption of Lead and Nickel by Biomass of Marine Algae,” Biotechnol. Bioeng., 43, 1001–1009 (1994).
Ho, Y.S., Comment on “Selective Adsorption of Tannins onto Hide Collagen Fibres,” Sci. China Ser. B: Chem., 48, 176 (2005).
Ho, Y.S. and G. McKay, “Pseudo-Second Order Model for Sorption Processes,” Process Biochem., 34, 451–465 (1999).
Ho, Y.S. and G. McKay, “Sorption of Dye from Aqueous Solution by Peat,” Chem. Eng. J., 70, 115–124 (1998).
Ho, Y.S. and G. McKay, “The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat,” Wat. Res., 3, 735–742 (2000).
Huang, J.P., C.P. Huang, and A.L. Morehart, “Removal of Cu (II) from Dilute Aqueous Solutions by Saccharomyces cerevisiae,” Wat. Res., 24(4), 433–499 (1990).
Iyer, A., K. Mody, and B. Jha, “Biosorption of Heavy Metals by a Marine Bacterium,” Mar. Pollut. Bull., 50(3), 340–343 (2005).
Kaewsarn, P., “Biosorption of Copper (II) from Aqueous Solutions by Pre-Treated Biomass of Marine Algae Padina sp.,” Chemosphere, 47, 1081–1085 (2002).
Kratochvil, D., B. Volesky, and G. Demopoulos, “Optimizing Cu Removal/Recovery in a Biosorption Column,” Wat. Res., 31(9), 2327–2339 (1997).
Kratochvil, D. and B. Volesky, “Biosorption of Cu from Ferruginous Wastewater by Algal Biomass,” Wat. Res., 32(9), 2760–2768 (1998).
Kuyucak, N. and B. Volesky, “Bioserbent for Recovery of Metals from Industrial Solutions,” Biotechnol. Lett., 10, 137–142 (1998).
Lewis, D. and R.J. Kriff, “The Removal of Heavy Metals from Aqueous Effluents by Immobilized Fungal Biomass,” Environ. Technol. Lett., 9, 991–998 (1988).
Lodeiro, P., B. Cordero, J.L. Barriada, R. Herrero, and M.E. Sastre de Vicente, “Biosorption of Cadmium by Biomass of Brown Marine Macroalgae,” Bioresource Technol., 96(16), 1796–1803 (2005).
Lo, W., H. Chua, K.H. Lam, and S.P. Bi, “A Comparative Investigation on the Biosorption of Lead by Filamentous Fungal Biomass,” Chemosphere, 39, 2723–2736 (1999).
Luo, F., Y. Liu, X. Li, Z. Xuan, and J. Ma, “Biosorption of Lead Ion by Chemically-Modified Biomass of Marine Brown Algae Laminaria japonica,” Chemosphere, 64(7), 1122–1127 (2006).
Martínez, M., N. Miralles, S. Hidalgo, N. Fiol, I. Villaescusa, and J. Poch, “Removal of Lead (II) and Cadmium (II) from Aqueous Solutions Using Grape Stalk Waste,” J. Hazard. Mater., 133(1–3), 203–211 (2006).
Matheickal, J.T. and Q. Yu, “Biosorption of Lead from Aqueous Solution by Macro-Fungi Phellinus badius,” in Proceedings of the 10th National Convention of Royal Australian Chemical Institute, Adelaide, Australia, 1996.
Matheickal, J.T., Q. Yu, and G.M. Woodburn, “Biosorption of Cadmium of Marine Algae Durvillaea potatorum,” Wat. Res., 33(2), 335–342 (1999).
Muraleedharan, T.R., L. Iyengar, and C. Venkobavhar, “Screening of Tropical Wood-Rotting Mushrooms for Copper Biosorption,” Appl. Environ. Microbiol., 61, 3507–3508 (1995).
Özer, A. and D. Özer, “Comparative of the Biosorption of Pb (II), Ni (II) and Cr (VI) Ions onto S. cerevisiae: Determination of Biosorption Heats,” J. Hazard. Mater., 100, 219–229 (2003).
Özer, A., D. Özer, and H. İbrahim Ekİz, “The Equilibrium and Kinetic Modelling of the Biosorption of Copper (II) Ions on Cladophora crispate,” Adso., 10(4), 317–326 (2004).
Özer, D., Z. Asksu, T. Kutsal, and A. Caglar, “Adsorption Isotherms of Lead (II) and Chromium (VI) on Cladophora crispat,” Environ. Technol., 15(5), 439–448 (1994).
Pal, A., S. Ghosh, and A.K. Paul, “Biosorption of Cobalt by Fungi from Serpentine Soil of Andaman,” Bioresource Technol., 97(10), 1253–1258 (2006).
Rao, P.S., S. Kalyani, K.V.N. Suresh Reddy, and A. Krishnaiah, “Comparison of Biosorption of Nickel (II) and Copper (II) Ions from Aqueous Solution by Sphaeroplea Algae and Acid Treated Sphaeroplea Algae,” Sep. Sci. Technol., 40(15), 3149–3165 (2005).
Sağ, Y. and T. Kutsal, “Determination of the Biosorption Heats of Heavy Metal Ions on Zooloea ramigera and Rhizopus arrhizus,” Biochem. Eng. J., 6, 145–151 (2000).
Sanchez, A., A. Balleste, M.L. Blazquez, F. González, J. Muñoz, and A. Hammaini, “Biosorption of Copper and Zinc by Cymodocea nodosa,” FEMS Microbiol. Rev., 23(5), 527–536 (1999).
Scott, J.A. and S.J. Palmer, “Sites of Cadmium Uptake in Bacteria Used for Biosorption,” Appl. Microbiol. Biotechnol., 33, 221–225 (1990).
Seki, H., A. Suzuki, and H. Maruyama, “Biosorption of Chromium (VI) and Arsenic (V) onto Methylated Yeast Biomass,” J. Colloid Interf. Sci., 281(2), 261–266 (2005).
Selatnia, A., A. Boukazoula, N. Kechid, M. Z. Bakhti, A. Chergui, and Y. Kerchich, “Biosorption of Lead (II) from Aqueous Solution by a Bacterial Dead Streptomyces rimosus Biomass,” Biochem. Eng. J., 19(2), 127–135 (2004).
Sheng, P.X., Y.P. Ting, J.P. Chen, and L. Hong, “Sorption of Lead, Copper, Cadmium, Zinc, and Nickel by Marine Algal Biomass: Characterization of Biosorptive Capacity and Investigation of Mechanisms,” J. Colloid Interf. Sci., 275(1), 131–141 (2004).
Singh, V.K. and P.N. Tiwari, “Removal and Recovery of Chromium (VI) from Industry Waste Water,” J. Chem. Technol. Biotechnol., 69, 376–382 (1997).
Smith, J.M., Chemical Engineering Kinetics, pp. 314–320, McGraw-Hill, Chemical Engineering Series, Singapore, 1981.
Suzuki, Y., T. Kametani, and T. Maruyama, “Removal of Heavy Metals from Aqueous Solution by Nonliving Ulva Seaweed as Biosorbent,” Wat. Res., 39(9), 1803–1808 (2005).
Terry P.A. and W. Stone, “Biosorption of Cadmium and Copper Contaminated Water by Scenedesmus abundans,” Chemosphere, 47(3), 249–255 (2002).
Treybal, R.E., Mass-Transfer Operations, pp. 566–575, McGraw-Hill, Singapore, 1980.
Tunali, S., T. Akar, A.S. Özcan, I. Kiran, and A. Özcan, “Equilibrium and Kinetics of Biosorption of Lead (II) from Aqueous Solutions by Cephalosporium aphidicola,” Sep. Purif. Technol., 47(3), 105–112 (2006).
Vijayaraghavan, K., J. Jegan, K. Palanivelu, and M. Velan, “Biosorption of Copper, Cobalt and Nickel by Marine Green Alga Ulva reticulata in a Packed Column,” Chemosphere, 60(3), 419–426 (2005).
Vijayaraghavan, K., T.V.N. Padmesh, K. Palanivelu, and M. Velan, “Biosorption of Nickel (II) Ions onto Sargassum wightii: Application of Two—Parameter and Three—Parameter Isotherm Models,” J. Hazard. Mater., 13(1–3), 304–308 (2006).
Villaescusa, I., N. Fiol, M. Martínez, N. Miralles, J. Poch, and J. Serarols, “Removal of Copper and Nickel Ions from Aqueous Solutions by Grape Stalks Wastes,” Wat. Res., 38, 992–1002 (2004).
Volesky, B., H. May, and Z. Holan, “Cadmium Biosorption by S. cereviceae,” Biotechnol. Bioeng., 41, 826–829 (1993).
Xue, H.B., W. Stumm, and L. Stagg, “The Binding of Heavy Metals to Algal Surfaces,” Wat. Res., 22, 917–926 (1988).
Yang, J. and B. Volesky, “Biosorption of Uranium on Sargassum Biomass,” Wat. Res., 33(15), 3357–3363 (1999).
Yu, Q., K. Pairat, W. Ma, T.M. Jose, and P. Yin, “Removal of Heavy Metal Ions from Wastewater by Using Biosorbents from Marine Algae—A Cost Effective New Technology,” Chinese J. of Chem. Eng., 9(2), 133–136 (2001).
Yu, Q., J.T. Matheickal, and P. Kaewsarn, “Heavy Metal Uptake Capacities of Common Marine Macro Algal Biomass,” Wat. Res., 33, 1534–1537 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, L., Su, Y., Su, H. et al. Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora fascicularis: Equilibrium, kinetics and environmental effects. Adsorption 12, 267–277 (2006). https://doi.org/10.1007/s10450-006-0503-y
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-006-0503-y