Abstract
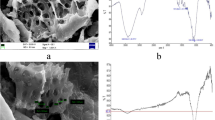
Utilization of one waste material to control pollution caused by another is of high significance in the remediation of environmental problems. Rice husk, an abundantly available agricultural waste, can be used as a low cost adsorbent for dyes and heavy metals in effluent streams. The possible utilization of rice husk ash as an adsorbent for methylene blue dye from aqueous solutions has been investigated. Ash samples from husks of two origins were prepared at different temperatures and their physical, chemical spectroscopic and morphological properties were determined. XRD, FTIR and SEM were some of the techniques adopted for the characterization. The samples were also analyzed for bulk density, pH, nitrogen adsorption properties and lime reactivity. Experiments of methylene blue adsorption on the ash samples were conducted using batch technique and a comparative study was made. Results were analyzed using linear, Langmuir and Freundlich isotherms. The values of separation factor indicate that most of the ash samples do adsorb the dye molecules, but in varying quantities. Calcination at 900∘C reduces the adsorption capacity of the ash to a great extent. Regression analysis shows that the experimental data fits both Langmuir and Freundlich isotherms for certain concentration limits. The adsorbate species are most probably transported from the bulk of the solution into the solid phase through intra-particle diffusion process. Kinetics of adsorption was found to follow pseudo second order rate equation with R 2∼ 0.99. The highest adsorption capacity (Q 0) achieved is found to be ∼690 mg/g, which is even higher than the values reported for activated carbon from rice husk. The adsorption capacity of the ash samples are in good agreement with their surface area and pore volume.
Similar content being viewed by others
References
Al-Degs, Y., M.A.M. Khraisheh, S.J. Allenm, and M.N. Ahmed, “Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent,” Water Research, 34, 927–935 (2000).
Allen, S.J., Q. Gan, R. Matthews, and P.A. Johnson, “Comparison of optimized isotherm models for basic dye adsorption,” Bioresoures Technology, 88, 143–52 (2003).
Annadurai, G., R.S. Juang, and D.J. Lee, “Use of cellulose-based wastes for adsorption of dyes from aqueous solutions,” Journal of Hazardous Material, B92, 263–274 (2002).
Bhattacharya, K.G. and Arunima Sarma, “Adsorption characteristics of the dye brilliant green on Neem leaf powder,” Dyes and Pigments, 57, 211–222 (2003).
Bhattacharya, K.G. and Sarma Arunima, “Kinetics and thermodynamics of methylene blueadsorption on Neem (Azadirachta indica) leaf powder,” Dyes and Pigments, 65, 51–59 (2005).
Chandrasekhar, S., P.N. Pramada, P. Raghavan, K.G. Satyanarayana, and T.N. Gupta, “Microsilica from rice husk as a possible substitute for condensed silica fume for high performance concrete,” Journal of Materials Science Letters, 21, 1245–1247 (2002).
Chandrasekhar, S., K.G. Satyanarayana, P.N. Pramada, P. Raghavan, and T.N. Gupta, “Review-Processing, properties and application of reactive silica from rice husk,” Journal of Materials Science, 21, 3159–3168 (2003).
Chandrasekhar, S., P.N. Pramada, and L. Praveen, “Effect of organic acid treatment on the properties of rice husk silica,” Journal of Materials Science, 40, 6535–6544 (2005).
Daifullah, A.A.M., B.S. Girgis, and H.M.H. Gad, “Utilization of agro-residues(rice husk)in small waste water treatment plans,” Materials Letters, 57, 1723–1731 (2003).
Dogan Mehment and Alkan Mohir, “Removal of MV from aqueous solution by Prlite,” Journal of Colloid and Interface Science, 267, 32–41 (2003).
Garg, V.K., M. Amita, Rakesh Kumar and Renuka Gupta, “Basic dye (methylene blue) removal from simulated waste water by adsorption using Indian rose wood saw dust: a timber industry waste,” Dyes and Pigments, 63, 243–250 (2004).
Ho, Y.S., “Citation review of Lagergren kinetic rate equation on adsorption reactions,” Scientometrics, 59, 171–177 (2004).
Ho, Y.S. and C.C. Chiang, “Sorption studies of acid dye by mixed sorbents,” Adsorption, 7, 139–147 (2001).
Ibrahim, D.M., S.A. El-Hemaly, and F.M. Abdel-Kerim, “Study of rice husk ash silica by IR spectroscopy,” Thermo Chimica Acta, 37, 307–14 (1980).
Kannan, N. and M. Meenakshi Sundaram, “Kinetics and mechanisam of removal of methylene blue by adsorption on various carbons-a comparitive study,” Dyes and Pigment, 51, 25–40 (2001).
Kannan, N. and M. Meenakshisundaram, “Adsorption of congo red on various activated carbons,” Water, Air and Soil Pollution, 138, 289–305 (2002).
Krishnarao, R.V., J. Subramanyam, and T. Jagadish Kumar, “Studies on formation of blackparticles in rice husk silica ash,” Journal of European Ceramic Society, 21, 99–104 (2001).
Low, K.S. and C.K. Lee, “Quaternized Rice husk as sorbent for reactive dyes,” Bioresource Technology, 61, 121–125 (1997).
Malik, P.K., “Use of activated carbons prepared from saw dust and rice husk for adsorption of acid dyes: a case study of acid yellow 36,” Dyes and Pigments, 56, 239–249 (2003).
Manaskorn Rachakornkij, Ruangchaay Sirawan, and Teachakulwiroj Sumate, “Removal of reactive dye from aqueous solution using bagasse fly ash,” Songklanakarin Journal of Science and Technology, 26, 13–24 (2004).
Mohamed, M.M., “Acid dye removal: A comparison of surfactant –modified mesoporous FSM-16 with activated carbon derived from rice husk,” Journal of Colloid and Interface Science, 272, 28–34 (2004).
Namasivayam, C., D. Prabha, and M. Kumutha, “Removal of direct red and acid brilliant blue by adsorption onto banana pith,” Bioresource Technology, 64, 77–79 (1998).
Namasivayam, C and D. Kavitha, “Removal of congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste,” Dyes and Pigments, 54, 47–58 (2002).
Rahman, I.A. and B. Saad, “Utilisation of Guava seeds as a source of activated carbon for removal of methyl blue from aqueous solution,” Malaysian Journal of Chemistry, 5, 8–14 (2003).
Sanghi, R. and B. Bhattacharya, “Review on decolorisation of aqueous dye solutions by low cost adsorbents,” Coloration Technology, 118, 256–269 (2002).
Senthilkumaar, S., P.R. Varadarajan, K. Porkodi, and C.V. Subbhuraam, “adsorption of methylene blue onto jute fibre carbon: kinetics and equilibrium studies,” Journal of Colloid and Interface Science, 284, 78–82 (2005).
Sumanjit and N. Prasad, “Adsorption of dyes on rice husk ash,” Indian Journal of Chemistry, 40A, 388–391 (2003).
Topallar, H. and Y. Bayrak, “Investigation of adsorption isotherms of myristic, palmitic and stearic acids on rice hull ash,” Turk Journal of Chemistry, 23, 193–198 (1999).
Vadivelan, V. and K. Vasanth Kumar, “Equilibrium, kinetics, mechanism and process design for the sorption of methylene blue onto rice husk,” Journal of Colloid and Interface Science, 286, 90–100 (2005).
Valix, M., W.H. Cheung, and G. Mckay, “Preparation of activated carbon using low temperature carbonization and physical activation of high ash raw bagasse for acid dye adsorption,” Chemosphere, 56, 493–501 (2004).
Voudrias, E., K. Fytianos, and E. Bonzani, “Sorption-Desorption isotherm of dyes from aqueous solutions and waste waters with different sorbent materials,” Globel Nest: The International Journal, 4, 75–83 (2002).
Waranusantigul, P., P. Pokethitiyook, M. Kruatrachue, and E.S. Upatham, “Kinetics of basic dye (Methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza),” Environmental Pollution, 125, 385–392 (2003).
Yupeng Guo, Zhang Hui, and Tao Nannan, “Adsorption of Malachite green and iodine on rice husk based porous carbon,” Materials Chemistry and Physics, 82, 107–15 (2003).
Yupeng Guo, Yang Shaofeng, Yu Karfeng, Zhao Jingzhe, Wang Zichen and Xu Hongding, “The preparation and mechanism studies of rice husk based porous carbon,” Materials Chemistry and Physics, 74, 320–323 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekhar, S., Pramada, P.N. Rice husk ash as an adsorbent for methylene blue—effect of ashing temperature. Adsorption 12, 27–43 (2006). https://doi.org/10.1007/s10450-006-0136-1
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-006-0136-1