Abstract
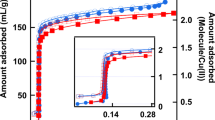
Confined water presents unusual properties in comparison with other sorbate species. First of all, the sorption isotherm is of type III, even in the microporous confinement range (Ø < 20 Å). Whatever the pore diameter, water sorption phenomenon looks like the so-called capillary condensation phase transition. Our results clearly valid such an expected behaviour in the mesoporous confinement range (20 Å < Ø < 40 Å). The water confined phase is a liquid phase characterized by a short range order and a high translational molecular mobility. The confinement induces a strong displacement towards the low temperature of the water confined liquid solidification Tsol. (for instance, Tsol. = 230 K for D2O confined liquid in MCM-41 (Ø = 24 Å). We have determined the structure of the water confined solid phase observed below Tsol.. It looks like those of the cubic ice structure affected by strong quasi-isotropic finite size effects induced by the confinement. Such a quasi-(1d) solid appears as a polycrystalline column rather than a single crystalline nanofiber. Concerning water confinement in the microporous range (as for example, AlPO4-5 zeolite (Ø = 7.3 Å)), our results are more surprising. Type III sorption isotherm is the signature of a crystallization phenomenon at room temperature (T = 300 K). The confined water crystallizes in two helices that are commensurate with the AlPO4-5 micropore structure. The confined ice has a density of 1.2 g⋅ cm− 3.
Similar content being viewed by others
References
Bellisent-Funel,M.-C., J., Lal, and L. Bosio, “Structural Study of Water Confined in Porous Glass by Neutron Scattering” J. Chem. Phys., 98, 4246–4252 (1993).
Choudhary, V.R., D.B., Akolekar, A.P. Singh, and S.D. Sansare, “Influence of Thermal, Hydrothermal, and Acid-Base Treatments on Structural Stability and Surface and Catalytic Properties of AlPO4-5” J. Catal., 111, 253–263 (1988).
Coulomb, J.P., N. Floquet, Y. Grillet, P.L. Llewellyn, R. Kahn, and G. Andrè, “Dynamic and Structural Properties of Confined Phases (Hydrogen, Methane and Water) in MCM-41 samples (1.9 nm, 2.5 nm and 4 nm)” Studies in Surface Science and Catalysis,128,235–242(2000).
Davis, M.E., C. Montes, P.E. Hathaway, J.P. Arhancet, D.L. Hasta, and J.M. Garces, “Physicochemical Properties of VP 1-5” J. Am. Chem. Soc., 111, 3919–3927 (1989).
Dore, J.C., “Structural Studies of Water in Confined Geometry by Neutron Diffraction” Chem. Phys., 258, 327–347 (2000).
Dunn, M., J.C., Dore, and P. Chieux, “Structural Studies of Ice Formation in Porous Silicas by Neutron Diffraction” J. Cryst. Growth, 92, 233–238 (1988).
Floquet, N., J.P., Coulomb, N. Dufau, and G. Andrè, “Structural and Dynamics of Confined Water in AlPO4-5 Zeolite” J. Phys. Chem. B., 108 13107–13115 (2004).
Floquet, N., J.P., Coulomb, N. Dufau, G. Andrè, and R. Kahn, “Structural and Dynamic Properties of Confined Water in Nanometric Model Porous Materials (8 Å ≤ Ø ≤ 40 Å)” Physica B, 350, 265–269 (2004).
Floquet, N., J.P. Coulomb, C. Martin, Y. Grillet, P.L. Llewellyn, and G. Andrè, “Neutron Diffraction Study of Phase Transitions observed during the Sorption of D2O on MCM-41 (40Å and 25Å)” in Proceedings of the 12th Int. Zeolite conference, M.J. Treacy et al., pp. 659–666, Material Research Society,1999.
Izmailova, S.G., E.A. Vasiljeva, I.V. Karetina, N.N. Feoktistova, and S.S. Khvoshchev, “Adsorption of Methanol, Ammonia and Water on the Zeolite-Like Aluminophosphates AIPO4-5, AIPO4-17, and AIPO4-18” J. Colloid Interface Sci., 179, 374–379 (1996).
Kolesnikov, A.I., V.V. Sinitsyn, E.G. Ponyatovsky, I. Natkaniec, L.S. Smirnov, and J.C. Li, “Neutron-Scattering Studies of Ice Prepared by Different Thermobaric Treatments” J. Phys. Chem., 101, 6082–6086 (1997).
Lohse, J., M., Noack, and E. Jahn, “Adsorption Properties of the AlPO4-5 Molecular Sieve” Adsorption Science and Technology, 3, 19–24 (1986).
Malla, P.B. and S., Komarneni, “Effect of Pore Size on the Chemical Removal of Organic Template Molecules from Synthetic Molecular Sieves” Zeolite, 15, 324–332 (1995).
Morishige, K. and K., Nobuoka, “X-ray Diffraction Studies of Freezing and Melting of Water Confined in a Mesoporous Adsorbent (MCM-41)” J. Chem. Phys 107, 6965–6969 (1997).
Newalkar, B.L., R.V., Jasra, V. Kamath, and S.G.T. Bhat, “Sorption of Water in Aluminophosphate Molecular Sieve AIPO4-5” Micropor. Mesopor. Mater., 20, 129–137 (1998).
Tsutsumi, K., K., Mizoe, and K. Chubachi, “Adsorption Characteristics and Surface Free Energy of AIPO4-5” Colloid Polym. Sci., 277, 83–88 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Floquet, N., Coulomb, J.P., Dufau, N. et al. Confined Water in Mesoporous MCM-41 and Nanoporous AlPO4-5: Structure and Dynamics. Adsorption 11 (Suppl 1), 139–144 (2005). https://doi.org/10.1007/s10450-005-5912-9
Issue Date:
DOI: https://doi.org/10.1007/s10450-005-5912-9