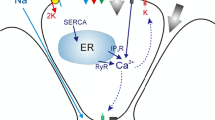
Abstract
Secretion is a fundamental cellular process involving the regulated release of intracellular products from cells. Physiological functions such as neurotransmission, or the release of hormones and digestive enzymes, are all governed by cell secretion. Anomalies in the processes involved in secretion contribute to the development and progression of diseases such as diabetes and other hormonal disorders. To unravel the mechanisms that govern such diseases, it is essential to understand how hormones, growth factors and neurotransmitters are synthesized and processed, and how their signals are recognized, amplified and transmitted by intracellular signaling pathways in the target cells. Here, we discuss diverse aspects of the detailed mechanisms involved in secretion based on mathematical models. The models range from stochastic ones describing the trafficking of secretory vesicles to deterministic ones investigating the regulation of cellular processes that underlie hormonal secretion. In all cases, the models are closely related to experimental results and suggest theoretical predictions for the secretion mechanisms.




Similar content being viewed by others
References
Atwater I, Dawson C, Scott A, Eddlestone G, Rojas E (1980) The nature of the oscillatory behaviour in electrical activity from pancreatic beta-cell. Horm Metab Res Suppl Suppl 10:100–107
Barg S, Rorsman P (2004) Insulin secretion: a high-affinity Ca2+ sensor after all? J Gen Physiol 124(6):623–625
Beauvois M, Merezak C, Jonas J, Ravier M, Henquin J, Gilon P (2006) Glucose-induced mixed \([\hbox{Ca}^2+]_{\rm c}\) oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2 + -ATPase of the endoplasmic reticulum. Am J Physiol-Cell Physiol 290(6):C1503–C1511
Ben-Jonathan N, Hnasko R (2004) Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22(6):724–763
Bertram R, Sherman A, Satin L (2007) Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol.-Endocrinol Metabol 293(4):E890
Bertuzzi A, Salinari S, Mingrone G (2007) Insulin granule trafficking in β-cells: mathematical model of glucose-induced insulin secretion. Am J Physiol.-Endocrinol Metabol 293(1):E396
Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson P, Rorsman P (2008) Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes 57(6):1618–1628
Burris T, Freeman M (1993) Low concentrations of dopamine increase cytosolic calcium in lactotrophs. Endocrinology 133(1):63–68
Cabrera O, Berman D, Kenyon N, Ricordi C, Berggren P, Caicedo A (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103(7):2334–2339
Cerasi E, Fick G, Rudemo M (1974) A mathematical model for the glucose induced insulin release in man. Eur J Clin Invest 4(4):267–278
Chen Y, Wang S, Sherman A (2008) Identifying the targets of the amplifying pathway for insulin secretion in pancreatic β-cells by kinetic modeling of granule exocytosis. Biophys J 95(5):2226–2241
Daniel S, Noda M, Straub S, Sharp G (1999) Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes 48(9):1686–1690
Denef C, Manet D, Dewals R (1980) Dopaminergic stimulation of prolactin release
Fridlyand L, Harbeck M, Roe M, Philipson L (2007) Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach. Am J Physiol-Cell Physiol 293(6):C1924
Grodsky G (1972) A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest 51(8):2047–2059
Holcman D, Schuss Z (2004) Escape through a small opening: Receptor trafficking in a synaptic membrane. J Stat Phys V 117(5):975–1014
Jonas J, Gilon P, Henquin J (1998) Temporal and quantitative correlations between insulin secretion and stably elevated or oscillatory cytoplasmic Ca2+ in mouse pancreatic β-cells. Diabetes 47(8):1266–1273
Jonkers F, Henquin J (2001) Measurements of cytoplasmic Ca2 + in islet cell clusters show that glucose rapidly recruits β-cells and gradually increases the individual cell response. Diabetes 50:540–550
Krauskopf B, Osinga H (2007) Computing invariant manifolds via the continuation of orbit segments. In: Krauskopf B, Osinga HM, Galán-Vioque J (eds) Numerical Continuation Methods for Dynamical Systems: Path following and boundary value problems. Springer-Verlag, pp 117–154
Licko V (1973) Threshold secretory mechanism: a model of derivative element in biological control. Bull Math Biol 35:51–58
Nesher R, Cerasi E (2002) Modeling phasic insulin release. Diabetes 51:53–58
Nowacki J, Mazlan SH, Osinga HM, Tsaneva-Atanasova KT (2010) The role of large-conductance calcium-activated K + (BK) channels in shaping bursting oscillations of a somatotroph cell model. Phys D 239(9):485–493
Olofsson C, Göpel S, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L (2002) Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic β-cells. Pflüg Archiv Eur J Physiol 444(1):43–51
Pedersen M (2009) Contributions of mathematical modeling of beta cells to the understanding of beta-cell oscillations and insulin secretion. J Diabet Sci Tech 3:12–20
Pedersen M, Sherman A (2009) Newcomer insulin secretory granules as a highly calcium-sensitive pool. Proc Natl Acad Sci USA 106(18):7432
Pedersen M, Corradin A, Toffolo G, Cobelli C (2008) A subcellular model of glucose-stimulated pancreatic insulin secretion. Phil Trans R Soc A 366(1880):3525
Pedersen M, Toffolo G, Cobelli C (2010) Cellular modeling: insight into oral minimal models of insulin secretion. Am J Phys-Endocrinol Metabol 298(3):E597
Schuss Z (1980) Theory and applications of stochastic differential equations. Wiley, New York
Sherman A, Rinzel J (1992) Rhythmogenic effects of weak electrotonic coupling in neuronal models. Proc Natl Acad Sci USA 89(6):2471–2474
Soria B, Andreu E, Berna G, Fuentes E, Gil A, León-Quinto T, Martín F, Montanya E, Nadal A, Reig J, et al (2000) Engineering pancreatic islets. Pflüg Archiv Eur J Physiol 440(1):1–18
Tabak J, Toporikova N, Freeman M, Bertram R (2007) Low dose of dopamine may stimulate prolactin secretion by increasing fast potassium currents. J Comput Neurosci 22(2):211–222
Toffolo G, Breda E, Cavaghan M, Ehrmann D, Polonsky K, Cobelli C (2001) Quantitative indexes of β-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol-Endocrinol Metabol 280(1):2–10
Toporikova N, Tabak J, Freeman M, Bertram R (2008) A-type K+ current can act as a trigger for bursting in the absence of a slow variable. Neural Comput 20(2):436–451
Trifaro J, Gasman S, Gutierrez L (2008) Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol 192(2):165–172
Tsaneva-Atanasova K, Burgo A, Galli T, Holcman D (2009) Quantifying neurite growth mediated by interactions among secretory vesicles, microtubules, and actin networks. Biophys J 96(3):840–857
Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic S (2007) Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol 98(1):131–144
van Goor F, Li Y, Stojilkovic S (2001a) Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci 21(16):5902–5915
van Goor F, Zivadinovic D, Martinez-Fuentes A, Stojilkovic S (2001b) Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. cell type-specific action potential secretion coupling. J Biol Chem 276(36):33840–33846
Wan Q, Dong Y, Yang H, Lou X, Ding J, Xu T (2004) Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+. J Gen Physiol 124(6):653–662
Yang Y, Gillis K (2004) A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol 124(6):641–651
Acknowledgments
KTA acknowledges funding from grant EP/E032249/1 of the Engineering and Physical Sciences Research Council (EPSRC). HMO was supported by an EPSRC Advanced Research Fellowship and an IGERT grant of the National Science Foundation. JT was supported by NIH grant DA-19356. MGP was supported by the Lundbeck Foundation and by the European Union through the Network of Excellence BioSim (contract no. LSHB-CT-2004-005137).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsaneva-Atanasova, K., Osinga, H.M., Tabak, J. et al. Modeling Mechanisms of Cell Secretion. Acta Biotheor 58, 315–327 (2010). https://doi.org/10.1007/s10441-010-9115-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-010-9115-8