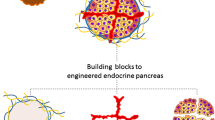
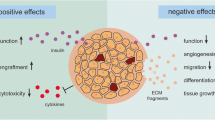
Abstract
Exosomes are enclosed within a single outer membrane and exemplify a specific subtype of secreted vesicles. Exosomes transfer signalling molecules, including microRNAs (miRNAs), messenger RNA (mRNA), fatty acids, proteins, and growth factors, making them a promising therapeutic tool. In routine bioartificial pancreas fabrication, cells are immobilized in polymeric hydrogels lacking attachment capability for cells and other biological cues. In this opinion article, we will discuss the potential role that exosomes and their specific biofactors may play to improve and sustain the function of this bioartificial construct. We will particularly discuss the challenges associated with their isolation and characterization. Since stem cells are an attractive source of exosomes, we will present the advantages of using exosomes in place of stem cells in medical devices including the bioartificial pancreas. We will provide literature evidence of active biofactors in exosomes to support their incorporation in the matrix of encapsulated islets. This will include their potential beneficial effect on hypoxic injury to encapsulated islets. In summary, we propose that the biofactors contained in secreted exosomes have significant potential to enhance the performance of islets encapsulated in polymeric material hydrogels with perm-selective properties to provide immunoisolation for islet transplants as an insulin delivery platform in diabetes.





Similar content being viewed by others
References
Aslan, C., S. H. Kiaie, N. M. Zolbanin, P. Lotfinejad, R. Ramezani, F. Kashanchi, and R. Jafari. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 21:20, 2021
Bagno, L., K. E. Hatzistergos, W. Balkan, and J. M. Hare. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 26(7):1610–1623, 2018. https://doi.org/10.1016/j.ymthe.2018.05.009
Balla, T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93(3):1019–1137, 2013
Bharadwaj, S., G. Liu, Y. Shi, C. Markert, K. E. Andersson, A. Atala, and Y. Zhang. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A. 17(15–16):2123–2132, 2011
Blanchard, N., D. Lankar, F. Faure, A. Regnault, C. Dumont, G. Raposo, and C. Hivroz. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 168(7):3235–3241, 2002
Chahar, H. S., X. Bao, and A. Casola. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 7(6):3204–3225, 2015
Chen, T. S., R. C. Lai, M. M. Lee, A. B. Choo, C. N. Lee, and S. K. Lim. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38(1):215–224, 2010
Colao, I. L., R. Corteling, D. Bracewell, and I. Wall. Manufacturing exosomes: a promising therapeutic platform. Trends Mol. Med. 24(3):242–256, 2018
Coughlan, C., K. D. Bruce, O. Burgy, T. D. Boyd, C. R. Michel, J. E. Garcia-Perez, V. Adame, P. Anton, B. M. Bettcher, H. J. Chial, M. Königshoff, E. Hsieh, M. Graner, and H. Potter. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell. Biol.88(1):e110, 2020
Denzer, K., M. J. Kleijmeer, H. F. Heijnen, W. Stoorvogel, and H. J. Geuze. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113(Pt 19):3365–3374, 2000
Doyle, L. M., and M. Z. Wang. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 8(7):727, 2019
Emamaullee, J. A., A. M. Shapiro, R. V. Rajotte, G. Korbutt, and J. F. Elliott. Neonatal porcine islets exhibit natural resistance to hypoxia-induced apoptosis. Transplantation. 82(7):945–952, 2006
Enck, K., R. Tamburrini, C. Deborah, C. Gazia, A. Jost, F. Khalil, A. Alwan, G. Orlando, and E. C. Opara. Effect of alginate matrix engineered to mimic the pancreatic microenvironment on encapsulated islet function. Biotechnol. Bioeng. 118(3):1177–1185, 2021
Escola, J. M., M. J. Kleijmeer, W. Stoorvogel, J. M. Griffith, O. Yoshie, and H. J. Geuze. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273(32):20121–20127, 1998
Gamble, A., R. Pawlick, A. R. Pepper, A. Bruni, A. Adesida, P. A. Senior, G. S. Korbutt, and A. Shapiro. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PLoS ONE.13(11):e0206449, 2018
Geuze, H. J. The role of endosomes and lysosomes in MHC class II functioning. Immunol. Today. 19(6):282–287, 1998
Heldring, N., I. Mäger, M. J. Wood, K. Le Blanc, and S. E. Andaloussi. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 26(8):506–517, 2015
Hong, P., S. Koza, and E. S. Bouvier. Size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J. Liq. Chromatogr. Relat. Technol. 35(20):2923–2950, 2012
Ikonen, E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13(4):470–477, 2001
Jan, A. T., S. Rahman, S. Khan, S. A. Tasduq, and I. Choi. Biology, pathophysiological role, and clinical implications of exosomes: a critical appraisal. Cells. 8(2):99, 2019
Jung, M. K., and J. Y. Mun. Sample preparation and imaging of exosomes by transmission electron microscopy. J. Vis. Exp. 131:56482, 2018
Kalluri, R., and V. S. LeBleu. The biology, function, and biomedical applications of exosomes. Science. 367(6478):6977, 2020
Kim, W., Y. Gwon, S. Park, H. Kim, and J. Kim. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 19:50–74, 2022
Kleijmeer, M., G. Ramm, D. Schuurhuis, J. Griffith, M. Rescigno, P. Ricciardi-Castagnoli, A. Y. Rudensky, F. Ossendorp, C. J. Melief, W. Stoorvogel, and H. J. Geuze. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol. 155(1):53–63, 2001
Lai, R. C., S. S. Tan, B. J. Teh, S. K. Sze, F. Arslan, D. P. de Kleijn, A. Choo, and S. K. Lim. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics. 1:971907, 2012
Li, P., M. Kaslan, S. H. Lee, J. Ya, and Z. Gao. Progress in exosome isolation techniques. Theranostics. 7(3):789–804, 2017. https://doi.org/10.7150/thno.18133
Lim, F., and A. M. Sun. Microencapsulated islets as bioartificial endocrine pancreas. Science. 210(4472):908–910, 1980
Liu, J., F. Jiang, Y. Jiang, Y. Wang, Z. Li, X. Shi, Y. Zhu, H. Wang, and Z. Zhang. Roles of exosomes in ocular diseases. Int. J. Nanomed. 15:10519–10538, 2020
Llacua, A., B. J. de Haan, S. A. Smink, and P. de Vos. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J. Biomed. Mater. Res. Part A. 104(7):1788–1796, 2016
Lou, G., Z. Chen, M. Zheng, and Y. Liu. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med.49(6):e346, 2017
Ludwig, N., T. L. Whiteside, and T. E. Reichert. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 20(19):4684, 2019
McQuilling, J. P., S. Sittadjody, S. Pendergraft, A. C. Farney, and E. C. Opara. Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. Biomater. Sci. 5(12):2437–2447, 2017
Mendt, M., S. Kamerkar, H. Sugimoto, K. M. McAndrews, C. C. Wu, M. Gagea, S. Yang, E. Blanko, Q. Peng, X. Ma, J. R. Marszalek, A. Maitra, C. Yee, K. Rezvani, E. Shpall, V. S. LeBleu, and R. Kalluri. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight.3(8):e99263, 2018
Opara, A., A. Jost, S. Dagogo-Jack, and E. C. Opara. Islet cell encapsulation - application in diabetes treatment. Exp. Boil. Med. (Maywood). 246(24):2570–2578, 2021
Pessolano, E., R. V. Bizzarro, P. Franco, I. Marco, F. Petrella, A. Porta, A. Tosco, L. Parente, M. Perretti, and A. Petrella. Annexin A1 contained in extracellular vesicles promotes the activation of keratinocytes by mesoglycan effects: an autocrine loop through FPRs. Cells. 8(7):753, 2019
Peters, P. J., J. Borst, V. Oorschot, M. Fukuda, O. Krähenbühl, J. Tschopp, J. W. Slot, and H. J. Geuze. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173(5):1099–1109, 1991
Rabesandratana, H., J. P. Toutant, H. Reggio, and M. Vidal. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during In vitro maturation of reticulocytes. Blood. 91(7):2573–2580, 1998
Rahman, M. J., D. Regn, R. Bashratyan, and Y. D. Dai. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes. 63(3):1008–1020, 2014
Raposo, G., H. W. Nijman, W. Stoorvogel, R. Liejendekker, C. V. Harding, C. J. Melief, and H. J. Geuze. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183(3):1161–1172, 1996
Saeedi, P., I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, L. Guariguata, A. A. Motala, K. Ogurtsova, J. E. Shaw, D. Bright, R. Williams, and I. D. F. DiabetesAtlasCommittee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas 9th edition. Diabetes Res. Clin. Pract. 157, 107843, 2019
Sidhom, K., P. O. Obi, and A. Saleem. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int. J. Mol. Sci. 21(18):6466, 2020
Stinchcombe, J. C., and G. M. Griffiths. Regulated secretion from hemopoietic cells. J. Cell Biol. 147(1):1–6, 1999
Théry, C., A. Regnault, J. Garin, J. Wolfers, L. Zitvogel, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147(3):599–610, 1999
Théry, C., M. Boussac, P. Véron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. Baltimore. Md. 166(12):7309–7318, 2001
Vidal, M., P. Mangeat, and D. Hoekstra. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J. Cell Sci. 110(Pt 16):1867–1877, 1997
Wu, R., M. Soland, G. Liu, Y. Shi, C. Zhang, Y. Tang, G. Almeida-Porada, and Y. Zhang. Functional characterization of the immunomodulatory properties of human urine-derived stem cells. Transl. Androl. Urol. 10(9):3566–3578, 2021
Wubbolts, R., M. Fernandez-Borja, L. Oomen, D. Verwoerd, H. Janssen, J. Calafat, A. Tulp, S. Dusseljee, and J. Neefjes. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J. Cell Biol. 135(3):611–622, 1996
Yang, Y., Y. Hong, E. Cho, G. B. Kim, and I. S. Kim. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles. 7(1):1440131, 2018
Yoshioka, Y., Y. Konishi, N. Kosaka, T. Katsuda, T. Kato, and T. Ochiya. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles. 2013. https://doi.org/10.3402/jev.v2i0.20424
Zaborowski, M. P., L. Balaj, X. O. Breakefield, and C. P. Lai. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 65(8):783–797, 2015
Zitvogel, L., A. Regnault, A. Lozier, J. Wolfers, C. Flament, D. Tenza, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4(5):594–600, 1998
Acknowledgments
The authors gratefully acknowledge financial support from the Wake Forest University Centre for Functional Materials.
Author Contributions
PC generated the manuscript draft with formatting; AA performed the experiments and provided the data shown in the manuscript and also assisted with manuscript writing; FK helped to design and supervise the experiments and helped with data interpretation; YZ performed the characterization of the urine-derived stem cells and provided guidance on the culture and use of the cells and helped with the revision of the manuscript; ECO conceived the idea and reviewed and edited the manuscript.
Conflict of interest
The authors disclose no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Canning, P., Alwan, A., Khalil, F. et al. Perspectives and Challenges on the Potential Use of Exosomes in Bioartificial Pancreas Engineering. Ann Biomed Eng 50, 1177–1186 (2022). https://doi.org/10.1007/s10439-022-03004-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-03004-0