Abstract
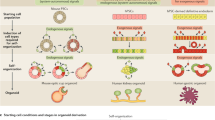
Recent advances in stem cell biology, synthetic biology, bioengineering, and biotechnology have included significant work leading to the development of stem cell-derived organoids. The growing popularity of organoid research and use of organoids is widely due to the fact that these three-dimensional cellular structures better model human physiology compared to traditional in vitro and in vivo methods by recapitulating many biologically relevant parameters. Organoids show great promise for a wide range of applications, such as for use in disease modeling, drug discovery, and regenerative medicine. However, many challenges associated with reproducibility and scale up still remain. Identification of the conditions which generate a robust environment that predictably promotes cellular self-assembly and organization leading to organoid formation is critical and requires a multidisciplinary approach. To accomplish this we need to identify a cellular source, engineer a matrix to stimulate cell–cell and cell–matrix interactions, and provide the biochemical and biophysical cues which mimic that of the in vivo environment. Discussion of the components needed for organoid development and formation is reviewed herein, as well as specific organoid examples and the promise of this research for the future.


Similar content being viewed by others
References
Allazetta, S., and M. P. Lutolf. Stem cell niche engineering through droplet microfluidics. Curr. Opin. Biotechnol. 35:86–93, 2015.
Annabi, N., A. Tamayol, S. R. Shin, A. M. Ghaemmaghami, N. A. Peppas, and A. Khademhosseini. Surgical materials: current challenges and nano-enabled solutions. Nano Today 9:574–589, 2014.
Barker, N., J. H. van Es, J. Kuipers, P. Kujala, M. van den Born, M. Cozijnsen, A. Haegebarth, J. Korving, H. Begthel, P. J. Peters, and H. Clevers. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007, 2007.
Bhatia, S. N., and D. E. Ingber. Microfluidic organs-on-chips. Nat. Biotechnol. 32:760–772, 2014.
Brassard, J. A., and M. P. Lutolf. Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24:860–876, 2019.
Broguiere, N., L. Isenmann, C. Hirt, T. Ringel, S. Placzek, E. Cavalli, F. Ringnalda, L. Villiger, R. Züllig, R. Lehmann, G. Rogler, M. H. Heim, J. Schüler, M. Zenobi-Wong, and G. Schwank. Growth of epithelial organoids in a defined hydrogel. Adv. Mater. 30:1801621, 2018.
Broutier, L., A. Andersson-Rolf, C. J. Hindley, S. F. Boj, H. Clevers, B. K. Koo, and M. Huch. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11:1724–1743, 2016.
Burdick, J. A., and G. Vunjak-Novakovic. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 15:205–219, 2009.
Capeling, M. M., M. Czerwinski, S. Huang, Y.-H. Tsai, A. Wu, M. S. Nagy, B. Juliar, N. Sundaram, Y. Song, W. M. Han, S. Takayama, E. Alsberg, A. J. Garcia, M. Helmrath, A. J. Putnam, and J. R. Spence. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 12:381–394, 2019.
Casey, J., X. Yue, T. D. Nguyen, A. Acun, V. R. Zellmer, S. Zhang, and P. Zorlutuna. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 12:025009, 2017.
Cha, C., W. B. Liechty, A. Khademhosseini, and N. A. Peppas. Designing biomaterials to direct stem cell fate. ACS Nano 6:9353–9358, 2012.
Chiu, L. L. Y., R. K. Iyer, J.-P. King, and M. Radisic. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng. A 17:1465–1477, 2011.
Choi, Y. Y., B. G. Chung, D. H. Lee, A. Khademhosseini, J.-H. Kim, and S.-H. Lee. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials 31:4296–4303, 2010.
Cruz-Acuña, R., M. Quirós, A. E. Farkas, P. H. Dedhia, S. Huang, D. Siuda, V. García-Hernández, A. J. Miller, J. R. Spence, A. Nusrat, and A. J. García. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19:1326–1335, 2017.
Devaud, Y. R., E. Avilla-Royo, C. Trachsel, J. Grossmann, I. Martin, M. P. Lutolf, and M. Ehrbar. Label-free quantification proteomics for the identification of mesenchymal stromal cell matrisome inside 3D poly(ethylene glycol) hydrogels. Adv. Healthc. Mater. 7:1800534, 2018.
Di Lullo, E., and A. R. Kriegstein. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18:573–584, 2017.
DiMarco, R. L., R. E. Dewi, G. Bernal, C. Kuo, and S. C. Heilshorn. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci. 3:1376–1385, 2015.
Discher, D. E., D. J. Mooney, and P. W. Zandstra. Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677, 2009.
Dutta, D., I. Heo, and H. Clevers. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23:393–410, 2017.
Dye, B. R., D. R. Hill, M. A. Ferguson, Y.-H. Tsai, M. S. Nagy, R. Dyal, J. M. Wells, C. N. Mayhew, R. Nattiv, O. D. Klein, E. S. White, G. H. Deutsch, and J. R. Spence. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015. https://doi.org/10.7554/eLife.05098.
Gjorevski, N., A. Ranga, and M. P. Lutolf. Bioengineering approaches to guide stem cell-based organogenesis. Development 141:1794–1804, 2014.
Gjorevski, N., N. Sachs, A. Manfrin, S. Giger, M. E. Bragina, P. Ordóñez-Morán, H. Clevers, and M. P. Lutolf. Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–564, 2016.
Guo, W. H., M. T. Frey, N. A. Burnham, and Y. L. Wang. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 90:2213–2220, 2006.
Hoang, P., J. Wang, B. R. Conklin, K. E. Healy, and Z. Ma. Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat. Protoc. 13:723–737, 2018.
Huch, M., C. Dorrell, S. F. Boj, J. H. Van Es, V. S. W. Li, M. Van De Wetering, T. Sato, K. Hamer, N. Sasaki, M. J. Finegold, A. Haft, R. G. Vries, M. Grompe, and H. Clevers. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494:247–250, 2013.
Hughes, C. S., L. M. Postovit, and G. A. Lajoie. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1890, 2010.
Huh, D., G. A. Hamilton, and D. E. Ingber. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21:745–754, 2011.
Hwang, Y.-S., B. G. Chung, D. Ortmann, N. Hattori, H.-C. Moeller, and A. Khademhosseini. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl Acad. Sci. USA 106:16978–16983, 2009.
Iyer, R. K., L. L. Y. Chiu, and M. Radisic. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J. Biomed. Mater. Res. A 89:616–631, 2009.
Karp, J. M., J. Yeh, G. Eng, J. Fukuda, J. Blumling, K.-Y. Suh, J. Cheng, A. Mahdavi, J. Borenstein, R. Langer, and A. Khademhosseini. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7:786–794, 2007.
Khademhosseini, A., G. Eng, J. Yeh, P. A. Kucharczyk, R. Langer, G. Vunjak-Novakovic, and M. Radisic. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices 9:149–157, 2007.
Lancaster, M. A., and J. A. Knoblich. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125, 2014.
Lancaster, M. A., M. Renner, C. A. Martin, D. Wenzel, L. S. Bicknell, M. E. Hurles, T. Homfray, J. M. Penninger, A. P. Jackson, and J. A. Knoblich. Cerebral organoids model human brain development and microcephaly. Nature 501:373–379, 2013.
Lindborg, B. A., J. H. Brekke, A. L. Vegoe, C. B. Ulrich, K. T. Haider, S. Subramaniam, S. L. Venhuizen, C. R. Eide, P. J. Orchard, W. Chen, Q. Wang, F. Pelaez, C. M. Scott, E. Kokkoli, S. A. Keirstead, J. R. Dutton, J. Tolar, and T. D. O’Brien. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl. Med. 5:970–979, 2016.
Matthys, O. B., T. A. Hookway, and T. C. McDevitt. Design principles for engineering of tissues from human pluripotent stem cells. Curr. Stem Cell Rep. 2:43–51, 2016.
McCracken, K. W., E. M. Catá, C. M. Crawford, K. L. Sinagoga, M. Schumacher, B. E. Rockich, Y.-H. Tsai, C. N. Mayhew, J. R. Spence, Y. Zavros, and J. M. Wells. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404, 2014.
McCracken, K. W., J. C. Howell, J. M. Wells, and J. R. Spence. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6:1920–1928, 2011.
Mori, R., Y. Sakai, and K. Nakazawa. Micropatterned organoid culture of rat hepatocytes and HepG2 cells. J. Biosci. Bioeng. 106:237–242, 2008.
Murrow, L. M., R. J. Weber, and Z. J. Gartner. Dissecting the stem cell niche with organoid models: an engineering-based approach. Development 144:998–1007, 2017.
Purwada, A., M. K. Jaiswal, H. Ahn, T. Nojima, D. Kitamura, A. K. Gaharwar, L. Cerchietti, and A. Singh. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials 63:24–34, 2015.
Purwada, A., S. B. Shah, W. Beguelin, A. M. Melnick, and A. Singh. Modular immune organoids with integrin ligand specificity differentially regulate ex vivo B cell activation. ACS Biomater. Sci. Eng. 3:214–225, 2017.
Purwada, A., and A. Singh. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc. 12:168–182, 2017.
Qian, X., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254, 2016.
Quadrato, G., J. Brown, and P. Arlotta. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 22:1220–1228, 2016.
Roch, A., S. Giger, M. Girotra, V. Campos, N. Vannini, O. Naveiras, S. Gobaa, and M. P. Lutolf. Single-cell analyses identify bioengineered niches for enhanced maintenance of hematopoietic stem cells. Nat. Commun. 8:221, 2017.
Rock, J. R., M. W. Onaitis, E. L. Rawlins, Y. Lu, C. P. Clark, Y. Xue, S. H. Randell, and B. L. M. Hogan. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106:12771–12775, 2009.
Rossi, G., A. Manfrin, and M. P. Lutolf. Progress and potential in organoid research. Nat. Rev. Genet. 19:671–687, 2018.
Sato, T., D. E. Stange, M. Ferrante, R. G. J. Vries, J. H. van Es, S. van den Brink, W. J. van Houdt, A. Pronk, J. van Gorp, P. D. Siersema, and H. Clevers. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772, 2011.
Sato, T., R. G. Vries, H. J. Snippert, M. van de Wetering, N. Barker, D. E. Stange, J. H. van Es, A. Abo, P. Kujala, P. J. Peters, and H. Clevers. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265, 2009.
Scadden, D. T. Nice neighborhood: emerging concepts of the stem cell niche. Cell 157:41–50, 2014.
Schultz, K. M., K. A. Kyburz, and K. S. Anseth. Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl Acad. Sci. USA 112:E3757–E3764, 2015.
Schwartz, M. P., Z. Hou, N. E. Propson, J. Zhang, C. J. Engstrom, V. Santos Costa, P. Jiang, B. K. Nguyen, J. M. Bolin, W. Daly, Y. Wang, R. Stewart, C. D. Page, W. L. Murphy, and J. A. Thomson. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl Acad. Sci. USA 112:12516–12521, 2015.
Shkumatov, A., K. Baek, and H. Kong. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS ONE 9:e94764, 2014.
Spence, J. R., C. N. Mayhew, S. A. Rankin, M. F. Kuhar, J. E. Vallance, K. Tolle, E. E. Hoskins, V. V. Kalinichenko, S. I. Wells, A. M. Zorn, N. F. Shroyer, and J. M. Wells. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109, 2011.
Wells, J. M., J. R. Spence, and N. M. Le Douarin. How to make an intestine. Development 141:752–760, 2014.
Yang, C., F. W. DelRio, H. Ma, A. R. Killaars, L. P. Basta, K. A. Kyburz, and K. S. Anseth. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl Acad. Sci. USA 113:E4439–E4445, 2016.
Yang, C., M. W. Tibbitt, L. Basta, and K. S. Anseth. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13:645–652, 2014.
Yin, X., B. E. Mead, H. Safaee, R. Langer, J. M. Karp, and O. Levy. Engineering stem cell organoids. Cell Stem Cell 18:25–38, 2016.
Acknowledgments
We acknowledge support from the National Institutes of Health under Award Number R01-EB022025. In addition, N.A.P. acknowledges support from the Cockrell Family Chair Foundation, the Office of the Dean of the Cockrell School of Engineering at the University of Texas at Austin (UT) for the Institute for Biomaterials, Drug Delivery, and Regenerative Medicine, and the UT-Portugal Collaborative Research Program. During this work, M.E.W. was supported by a National Science Foundation Graduate Research Fellowship (DGE-1610403).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wechsler, M.E., Shevchuk, M. & Peppas, N.A. Developing a Multidisciplinary Approach for Engineering Stem Cell Organoids. Ann Biomed Eng 48, 1895–1904 (2020). https://doi.org/10.1007/s10439-019-02391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02391-1