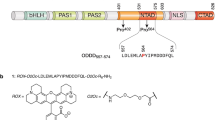
Abstract
Hypoxia is a typical hallmark of various diseases, including cancer, ischemic diseases, and stroke. It is also associated with the disease progression. Therefore, it is critical to develop an effective strategy to target the hypoxic region for diagnosis and treatment. In this review, we summarize recent progress in the development of hypoxia-responsive systems for imaging, sensing and therapy. Two types of hypoxia-sensitive systems, the hypoxia inducible factor-1 based systems and bioreductive molecule based systems, were reviewed with comments on their advantages and limitations. Future opportunities and challenges are also discussed in the end.







Similar content being viewed by others
References
Abbattista, M. R., S. M. Jamieson, Y. Gu, J. E. Nickel, S. M. Pullen, A. V. Patterson, W. R. Wilson, and C. P. Guise. Pre-clinical activity of PR-104 as monotherapy and in combination with sorafenib in hepatocellular carcinoma. Cancer Biol. Ther. 16:610–622, 2015.
Ahn, G.-O., K. J. Botting, A. V. Patterson, D. C. Ware, M. Tercel, and W. R. Wilson. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt (III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem. Pharmacol. 71:1683–1694, 2006.
Arany, Z., S.-Y. Foo, Y. Ma, J. L. Ruas, A. Bommi-Reddy, G. Girnun, M. Cooper, D. Laznik, J. Chinsomboon, and S. M. Rangwala. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1&agr. Nature 451:1008–1012, 2008.
Bhang, S. H., J. H. Kim, H. S. Yang, W.-G. La, T.-J. Lee, G. H. Kim, H. A. Kim, M. Lee, and B.-S. Kim. Combined gene therapy with hypoxia-inducible factor-1α and heme oxygenase-1 for therapeutic angiogenesis. Tissue Eng. Part A 17:915–926, 2010.
Binley, K., Z. Askham, L. Martin, H. Spearman, D. Day, S. Kingsman, and S. Naylor. Hypoxia-mediated tumour targeting. Gene Ther. 10:540–549, 2003.
Borad, M. J., S. G. Reddy, N. Bahary, H. E. Uronis, D. Sigal, A. L. Cohn, W. R. Schelman, J. Stephenson, E. G. Chiorean, and P. J. Rosen. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 33:1475–1481, 2015.
Bowers, D. T., M. L. Tanes, A. Das, Y. Lin, N. A. Keane, R. A. Neal, M. E. Ogle, K. L. Brayman, C. L. Fraser, and E. A. Botchwey. Spatiotemporal oxygen sensing using dual emissive boron dye-polylactide nanofibers. ACS Nano 8:12080–12091, 2014.
Brown, J. M., and W. R. Wilson. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4:437–447, 2004.
Bruehlmeier, M., U. Roelcke, P. A. Schubiger, and S. M. Ametamey. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J. Nucl. Med. 45:1851–1859, 2004.
Carreau, A., B. E. Hafny-Rahbi, A. Matejuk, C. Grillon, and C. Kieda. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 15:1239–1253, 2011.
Choi, B. H., Y. Ha, C.-H. Ahn, X. Huang, J.-M. Kim, S. R. Park, H. Park, H. C. Park, S. W. Kim, and M. Lee. A hypoxia-inducible gene expression system using erythropoietin 3′ untranslated region for the gene therapy of rat spinal cord injury. Neurosci. Lett. 412:118–122, 2007.
Coleman, C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J. Natl. Cancer Inst. 80:310–317, 1988.
Cui, L., Y. Zhong, W. Zhu, Y. Xu, Q. Du, X. Wang, X. Qian, and Y. Xiao. A new prodrug-derived ratiometric fluorescent probe for hypoxia: high selectivity of nitroreductase and imaging in tumor cell. Org. Lett. 13:928–931, 2011.
Cullberg, K. B., J. Olholm, S. K. Paulsen, C. B. Foldager, M. Lind, B. Richelsen, and S. B. Pedersen. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur. J. Pharm. Sci. 49:251–257, 2013.
Dachs, G. U., A. V. Patterson, J. D. Firth, P. J. Ratcliffe, K. S. Townsend, I. J. Stratford, and A. L. Harris. Targeting gene expression to hypoxic tumor cells. Nat. Med. 3:515–520, 1997.
Di Gregorio, E., G. Ferrauto, E. Gianolio, S. Lanzardo, C. Carrera, F. Fedeli, and S. Aime. An MRI method to map tumor hypoxia using red blood cells loaded with a pO2-responsive Gd-agent. ACS Nano 9:8239–8248, 2015.
Di, J., J. Yu, Y. Ye, D. Ranson, A. Jindal, and Z. Gu. Engineering synthetic insulin-secreting cells using hyaluronic acid microgels integrated with glucose-responsive nanoparticles. Cell. Mol. Bioeng. 8:445–454, 2015.
Do, Q. N., J. S. Ratnakar, Z. Kovács, and A. D. Sherry. Redox-and hypoxia-responsive MRI Contrast agents. ChemMedChem 9:1116–1129, 2014.
Duan, J.-X., H. Jiao, J. Kaizerman, T. Stanton, J. W. Evans, L. Lan, G. Lorente, M. Banica, D. Jung, and J. Wang. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 51:2412–2420, 2008.
Edgar, L. J., R. N. Vellanki, A. Halupa, D. Hedley, B. G. Wouters, and M. Nitz. Identification of hypoxic cells using an organotellurium tag compatible with mass cytometry. Angew. Chem. Int. Ed. 53:11473–11477, 2014.
Eschmann, S.-M., F. Paulsen, M. Reimold, H. Dittmann, S. Welz, G. Reischl, H.-J. Machulla, and R. Bares. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J. Nucl. Med. 46:253–260, 2005.
Everett, S. A., E. Swann, M. A. Naylor, M. R. Stratford, K. B. Patel, N. Tian, R. G. Newman, B. Vojnovic, C. J. Moody, and P. Wardman. Modifying rates of reductive elimination of leaving groups from indolequinone prodrugs: a key factor in controlling hypoxia-selective drug release. Biochem. Pharmacol. 63:1629–1639, 2002.
Guise, C. P., A. M. Mowday, A. Ashoorzadeh, R. Yuan, W.-H. Lin, D.-H. Wu, J. B. Smaill, A. V. Patterson, and K. Ding. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin. J. Cancer 33:80, 2014.
Haffty, B. G., Y. H. Son, C. T. Sasaki, R. Papac, D. Fischer, S. Rockwell, A. Sartorelli, and J. J. Fischer. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck: results from two randomized clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 27:241–250, 1993.
Haffty, B. G., Y. H. Son, L. D. Wilson, R. Papac, D. Fischer, S. Rockwell, A. C. Sartorelli, D. Ross, C. T. Sasaki, and J. J. Fischer. Bioreductive alkylating agent porfiromycin in combination with radiation therapy for the management of squamous cell carcinoma of the head and neck. Radiat. Oncol. Investig. 5:235–245, 1997.
Haffty, B. G., L. D. Wilson, Y. H. Son, E. I. Cho, R. J. Papac, D. B. Fischer, S. Rockwell, A. C. Sartorelli, D. A. Ross, and C. T. Sasaki. Concurrent chemo-radiotherapy with mitomycin C compared with porfiromycin in squamous cell cancer of the head and neck: final results of a randomized clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 61:119–128, 2005.
Harris, A. L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2:38–47, 2002.
Hendricksen, K., E. Cornel, T. de Reijke, H. Arentsen, S. Chawla, and J. Witjes. Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J. Urol. 187:1195–1199, 2012.
Hodgkiss, R. J. Use of 2-nitroimidazoles as bioreductive markers for tumour hypoxia. Anti-Cancer Drug Des. 13:687–702, 1998.
Hong, S. W., J. W. Yoo, H. S. Kang, S. Kim, and D.-K. Lee. HIF-1α-dependent gene expression program during the nucleic acid-triggered antiviral innate immune responses. Mol. Cells 27:243–250, 2009.
Hu, Q., P. S. Katti, and Z. Gu. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 6:12273–12286, 2014.
Hu Q., Sun W., Wang C., Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2015.
Huang, B., A. Desai, S. Tang, T. P. Thomas, and J. R. Baker, Jr. The synthesis of ac (RGDyK) targeted SN38 prodrug with an indolequinone structure for bioreductive drug release. Org. Lett. 12:1384–1387, 2010.
Huang, L. E., J. Gu, M. Schau, and H. F. Bunn. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 95:7987–7992, 1998.
Inman, B. A., P. R. Stauffer, O. A. Craciunescu, P. F. Maccarini, M. W. Dewhirst, and Z. Vujaskovic. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int. J. Hyperth. 30:171–175, 2014.
Jiang, B.-H., E. Rue, G. L. Wang, R. Roe, and G. L. Semenza. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271:17771–17778, 1996.
Jiang, B.-H., G. L. Semenza, C. Bauer, and H. H. Marti. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271:1172–1180, 1996.
Jiang, B.-H., J. Z. Zheng, S. W. Leung, R. Roe, and G. L. Semenza. Transactivation and inhibitory domains of hypoxia-inducible factor 1α modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 272:19253–19260, 1997.
Kennedy, K. A., S. Rockwell, and A. C. Sartorelli. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 40:2356–2360, 1980.
Kim, H. A., K. Kim, S. W. Kim, and M. Lee. Transcriptional and post-translational regulatory system for hypoxia specific gene expression using the erythropoietin enhancer and the oxygen-dependent degradation domain. J. Controll. Release 121:218–224, 2007.
Kim, H. A., S. Lim, H.-H. Moon, S. W. Kim, K.-C. Hwang, M. Lee, S. H. Kim, and D. Choi. Hypoxia-inducible vascular endothelial growth factor gene therapy using the oxygen-dependent degradation domain in myocardial ischemia. Pharm. Res. 27:2075–2084, 2010.
Kiyose, K., K. Hanaoka, D. Oushiki, T. Nakamura, M. Kajimura, M. Suematsu, H. Nishimatsu, T. Yamane, T. Terai, and Y. Hirata. Hypoxia-sensitive fluorescent probes for in vivo real-time fluorescence imaging of acute ischemia. J. Am. Chem. Soc. 132:15846–15848, 2010.
Kizaka-Kondoh, S., and H. Konse-Nagasawa. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 100:1366–1373, 2009.
Kuchimaru, T., T. Kadonosono, S. Tanaka, T. Ushiki, M. Hiraoka, and S. Kizaka-Kondoh. In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system. PLoS One 5:e15736, 2010.
Lee, M., M. Bikram, S. Oh, D. A. Bull, and S. W. Kim. Sp1-dependent regulation of the RTP801 promoter and its application to hypoxia-inducible VEGF plasmid for ischemic disease. Pharm. Res. 21:736–741, 2004.
Lee, M., D. Choi, M. J. Choi, J. H. Jeong, W. J. Kim, S. Oh, Y.-H. Kim, D. A. Bull, and S. W. Kim. Hypoxia-inducible gene expression system using the erythropoietin enhancer and 3′-untranslated region for the VEGF gene therapy. J. Controll. Release 115:113–119, 2006.
Lee, M., E. S. Lee, Y. S. Kim, B. H. Choi, S. R. Park, H. S. Park, H. C. Park, S. W. Kim, and Y. Ha. Ischemic injury-specific gene expression in the rat spinal cord injury model using hypoxia-inducible system. Spine 30:2729–2734, 2005.
Lee, N. Y., J. G. Mechalakos, S. Nehmeh, Z. Lin, O. D. Squire, S. Cai, K. Chan, P. B. Zanzonico, C. Greco, and C. C. Ling. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 70:2–13, 2008.
Lee, M., J. Rentz, M. Bikram, S. Han, D. Bull, and S. Kim. Hypoxia-inducible VEGF gene delivery to ischemic myocardium using water-soluble lipopolymer. Gene Ther. 10:1535–1542, 2003.
Li, W., Y. Li, S. Guan, J. Fan, C. F. Cheng, A. M. Bright, C. Chinn, M. Chen, and D. T. Woodley. Extracellular heat shock protein-90α: linking hypoxia to skin cell motility and wound healing. EMBO J. 26:1221–1233, 2007.
Li, Y., Y. Sun, J. Li, Q. Su, W. Yuan, Y. Dai, C. Han, Q. Wang, W. Feng, and F. Li. Ultrasensitive near-infrared fluorescence-enhanced probe for in vivo nitroreductase imaging. J. Am. Chem. Soc. 137:6407–6416, 2015.
Lin, Q., C. Bao, Y. Yang, Q. Liang, D. Zhang, S. Cheng, and L. Zhu. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv. Mater. 25:1981–1986, 2013.
Liu, J., Y. Liu, W. Bu, J. Bu, Y. Sun, J. Du, and J. Shi. Ultrasensitive nanosensors based on upconversion nanoparticles for selective hypoxia imaging in vivo upon near-infrared excitation. J. Am. Chem. Soc. 136:9701–9709, 2014.
Liu, Q., J. D. Sun, J. Wang, D. Ahluwalia, A. F. Baker, L. D. Cranmer, D. Ferraro, Y. Wang, J.-X. Duan, and W. S. Ammons. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother. Pharmacol. 69:1487–1498, 2012.
Liu, H., R. Zhang, Y. Niu, Y. Li, C. Qiao, J. Weng, J. Li, X. Zhang, Z. Xiao, and X. Zhang. Development of hypoxia-triggered prodrug micelles as doxorubicin carriers for tumor therapy. RSC Adv. 5:20848–20857, 2015.
Lu, Y., W. Sun, and Z. Gu. Stimuli-responsive nanomaterials for therapeutic protein delivery. J. Controll. Release 194:1–19, 2014.
Manesh, D. M., J. El-Hoss, K. Evans, J. Richmond, C. E. Toscan, L. S. Bracken, A. Hedrick, R. Sutton, G. M. Marshall, and W. R. Wilson. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood 126:1193–1202, 2015.
McKeage, M. J., Y. Gu, W. R. Wilson, A. Hill, K. Amies, T. J. Melink, and M. B. Jameson. A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC Cancer 11:432, 2011.
Mitragotri, S., D. G. Anderson, X. Chen, E. K. Chow, D. Ho, A. V. Kabanov, J. M. Karp, K. Kataoka, C. A. Mirkin, and S. H. Petrosko. Accelerating the translation of nanomaterials in biomedicine. ACS Nano 9:6644–6654, 2015.
Nunn, A., K. Linder, and H. W. Strauss. Nitroimidazoles and imaging hypoxia. Eur. J. Nucl. Med. 22:265–280, 1995.
Patterson, A. V., D. M. Ferry, S. J. Edmunds, Y. Gu, R. S. Singleton, K. Patel, S. M. Pullen, K. O. Hicks, S. P. Syddall, and G. J. Atwell. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin. Cancer Res. 13:3922–3932, 2007.
Perche, F., S. Biswas, T. Wang, L. Zhu, and V. Torchilin. Hypoxia-targeted siRNA delivery. Angew. Chem. 126:3430–3434, 2014.
Phillips, R. M., H. R. Hendriks, and G. J. Peters. EO9 (Apaziquone): from the clinic to the laboratory and back again. Br. J. Pharmacol. 168:11–18, 2013.
Plumb, J. A., and P. Workman. Unusually marked hypoxic sensitization to indoloquinone E09 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int. J. Cancer 56:134–139, 1994.
Qian, C., J. Yu, Y. Chen, Q. Hu, X. Xiao, W. Sun, C. Wang, P. Feng, Q. Shen, and Z. Gu. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv. Mater. 2016. doi:10.1002/adma.201505869.
Rasey, J. S., P. D. Hofstrand, L. K. Chin, and T. J. Tewson. Characterization of [18F] fluoroetanidazole, a new radiopharmaceutical for detecting tumor hypoxia. J. Nucl. Med. 40:1072, 1999.
Rhim, T., D. Y. Lee, and M. Lee. Hypoxia as a target for tissue specific gene therapy. J. Controll. Release 172:484–494, 2013.
Rockwell, S., S. R. Keyes, and A. C. Sartorelli. Preclinical studies of porfiromycin as an adjunct to radiotherapy. Radiat. Res. 116:100–113, 1988.
Rojas-Quijano, F. A., G. Tircsó, E. Tircsóné Benyó, Z. Baranyai, H. Tran Hoang, F. K. Kálmán, P. K. Gulaka, V. D. Kodibagkar, S. Aime, and Z. Kovács. Synthesis and characterization of a hypoxia-sensitive MRI probe. Chem. Eur. J. 18:9669–9676, 2012.
Schoonman, G. G., P. S. Sándor, A. C. Nirkko, T. Lange, T. Jaermann, U. Dydak, C. Kremer, M. D. Ferrari, P. Boesiger, and R. W. Baumgartner. Hypoxia-induced acute mountain sickness is associated with intracellular cerebral edema: a 3 T magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 28:198–206, 2008.
Semenza, G. L., P. H. Roth, H.-M. Fang, and G. L. Wang. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269:23757–23763, 1994.
Shibata, T., N. Akiyama, M. Noda, K. Sasai, and M. Hiraoka. Enhancement of gene expression under hypoxic conditions using fragments of the human vascular endothelial growth factor and the erythropoietin genes. Int. J. Radiat. Oncol. Biol. Phys. 42:913–916, 1998.
Shibata, T., A. Giaccia, and J. Brown. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 7:493–498, 2000.
Singleton, R. S., C. P. Guise, D. M. Ferry, S. M. Pullen, M. J. Dorie, J. M. Brown, A. V. Patterson, and W. R. Wilson. DNA cross-links in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism, and cytotoxicity. Cancer Res. 69:3884–3891, 2009.
Sun, J. D., Q. Liu, J. Wang, D. Ahluwalia, D. Ferraro, Y. Wang, J.-X. Duan, W. S. Ammons, J. G. Curd, and M. D. Matteucci. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin. Cancer Res. 18:758–770, 2012.
Sun, W., Y. Lu, and Z. Gu. Advances in anticancer protein delivery using micro-/nanoparticles. Part. Syst. Charact. 31:1204–1222, 2014.
Tai, W., R. Mo, J. Di, V. Subramanian, X. Gu, J. B. Buse, and Z. Gu. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules 15:3495–3502, 2014.
Takasawa, M., R. R. Moustafa, and J.-C. Baron. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke 39:1629–1637, 2008.
Tanabe, K., H. Harada, M. Narazaki, K. Tanaka, K. Inafuku, H. Komatsu, T. Ito, H. Yamada, Y. Chujo, and T. Matsuda. Monitoring of biological one-electron reduction by 19F NMR using hypoxia selective activation of an 19F-labeled indolequinone derivative. J. Am. Chem. Soc. 131:15982–15983, 2009.
Thomson, P. I., V. L. Camus, Y. Hu, and C. J. Campbell. Series of quinone-containing nanosensors for biologically relevant redox potential determination by surface-enhanced Raman spectroscopy. Anal. Chem. 87:4719–4725, 2015.
Tomasz, M., and Y. Palom. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 76:73–87, 1997.
Tracy, J. W., and L. T. Webster. Drugs used in the chemotherapy of protozoal infections. The pharmacological basis of therapeutics (9th ed.). New York: McGraw-Hill Book Co., pp. 987–1008, 1996.
Tsuzuki, Y., D. Fukumura, B. Oosthuyse, C. Koike, P. Carmeliet, and R. K. Jain. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1α → hypoxia response element → VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 60:6248–6252, 2000.
van der Heijden, A. G., P. M. Moonen, E. B. Cornel, H. Vergunst, T. M. de Reijke, E. van Boven, E. J. Barten, R. Puri, C. K. van Kalken, and J. A. Witjes. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J. Urol. 176:1349–1353, 2006.
Wang, G. L., B.-H. Jiang, E. A. Rue, and G. L. Semenza. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510–5514, 1995.
Wang, G. L., and G. L. Semenza. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. 90:4304–4308, 1993.
Wang, X.-D., J. A. Stolwijk, T. Lang, M. Sperber, R. J. Meier, J. Wegener, and O. S. Wolfbeis. Ultra-small, highly stable, and sensitive dual nanosensors for imaging intracellular oxygen and pH in cytosol. J. Am. Chem. Soc. 134:17011–17014, 2012.
Ware, D. C., B. D. Palmer, W. R. Wilson, and W. A. Denny. Hypoxia-selective antitumor agents. 7. Metal complexes of aliphatic mustards as a new class of hypoxia-selective cytotoxins. Synthesis and evaluation of cobalt (III) complexes of bidentate mustards. J. Med. Chem. 36:1839–1846, 1993.
Weiss, G. J., J. R. Infante, E. G. Chiorean, M. J. Borad, J. C. Bendell, J. R. Molina, R. Tibes, R. K. Ramanathan, K. Lewandowski, and S. F. Jones. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin. Cancer Res. 17:2997–3004, 2011.
Won, Y.-W., M. Lee, H. A. Kim, D. A. Bull, and S. W. Kim. Post-translational regulated and hypoxia-responsible VEGF plasmid for efficient secretion. J. Controll. Release 160:525–531, 2012.
Ye, Y., J. Yu, N.-Y. Nguyen, J. B. Buse, and Z. Gu. Microneedle integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv. Mater. 2016. doi:10.1002/adma.201506025.
Yockman, J., D. Choi, M. Whitten, C. Chang, A. Kastenmeier, H. Erickson, A. Albanil, M. Lee, S. Kim, and D. Bull. Polymeric gene delivery of ischemia-inducible VEGF significantly attenuates infarct size and apoptosis following myocardial infarct. Gene Ther. 16:127–135, 2009.
Yu, J., Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse, and Z. Gu. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 112:8260–8265, 2015.
Yuan, J., Y.-Q. Xu, N.-N. Zhou, R. Wang, X.-H. Qian, and Y.-F. Xu. A highly selective turn-on fluorescent probe based on semi-cyanine for the detection of nitroreductase and hypoxic tumor cell imaging. RSC Adv. 4:56207–56210, 2014.
Zhang, G., G. M. Palmer, M. W. Dewhirst, and C. L. Fraser. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 8:747–751, 2009.
Zhang, Z., J. Yan, Y. Chang, S. S. Yan, and H. Shi. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem. 18:4335, 2011.
Zhao, Y., S. Wu, J. Wu, P. Jia, S. Gao, X. Yan, and Y. Wang. Introduction of hypoxia-targeting p53 fusion protein for the selective therapy of non-small cell lung cancer. Cancer Biol. Ther. 11:95–107, 2011.
Zheng, X., H. Tang, C. Xie, J. Zhang, W. Wu, and X. Jiang. Tracking cancer metastasis in vivo by using an iridium-based hypoxia-activated optical oxygen nanosensor. Angew. Chem. 127:8212–8217, 2015.
Zheng, X., X. Wang, H. Mao, W. Wu, B. Liu, and X. Jiang. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015. doi:10.1038/ncomms6834.
Zheng, R., Q. Yao, G. Xie, S. Du, C. Ren, Y. Wang, and Y. Yuan. TAT-ODD-p53 enhances the radiosensitivity of hypoxic breast cancer cells by inhibiting Parkin-mediated mitophagy. Oncotarget 6:17417–17429, 2015.
Zhou, S., L. Gu, J. He, H. Zhang, and M. Zhou. MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol. Cell. Biol. 31:4928–4937, 2011.
Zhu, H., and H. F. Bunn. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir. Physiol. 115:239–247, 1999.
Acknowledgment
This work was supported by the Grants from the American Diabetes Association (ADA) to Z.G. (1-14-JF-29 and 1-15-ACE-21) and the Grant from NC TraCS, NIH’s Clinical and Translational Science Awards (CTSA, NIH Grant 1UL1TR001111) at UNC-CH.
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Akhilesh K. Gaharwar oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Yu, J., Zhang, Y., Hu, X. et al. Hypoxia-Sensitive Materials for Biomedical Applications. Ann Biomed Eng 44, 1931–1945 (2016). https://doi.org/10.1007/s10439-016-1578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1578-6