Abstract
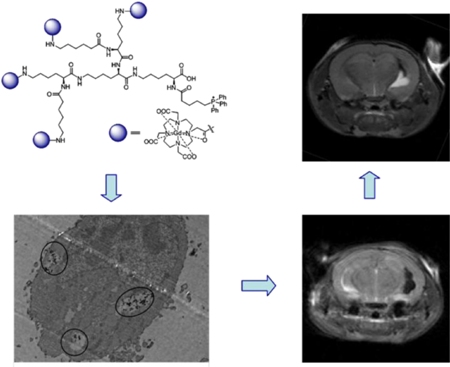
Cell therapy represents a promising therapeutic for a myriad of medical conditions, including cancer, traumatic brain injury, and cardiovascular disease among others. A thorough understanding of the efficacy and cellular dynamics of these therapies necessitates the ability to non-invasively track cells in vivo. Magnetic resonance imaging (MRI) provides a platform to track cells as a non-invasive modality with superior resolution and soft tissue contrast. We recently reported a new nanoprobe platform for cell labeling and imaging using fluorophore doped siloxane core nanoemulsions as dual modality (1H MRI/Fluorescence), dual-functional (oximetry/detection) nanoprobes. Here, we successfully demonstrate the labeling, dual-modality imaging, and oximetry of neural progenitor/stem cells (NPSCs) in vitro using this platform. Labeling at a concentration of 10 μL/104 cells with a 40%v/v polydimethylsiloxane core nanoemulsion, doped with rhodamine, had minimal effect on viability, no effect on migration, proliferation and differentiation of NPSCs and allowed for unambiguous visualization of labeled NPSCs by 1H MR and fluorescence and local pO2 reporting by labeled NPSCs. This new approach for cell labeling with a positive contrast 1H MR probe has the potential to improve mechanistic knowledge of current therapies, and guide the design of future cell therapies due to its clinical translatability.







Similar content being viewed by others
References
Addington, C. P., C. M. Pauken, M. R. Caplan, and S. E. Stabenfeldt. The role of SDF-1α-ECM crosstalk in determining neural stem cell fate. Biomaterials 35:3263–3272, 2014.
Ahrens, E. T., R. Flores, H. Xu, and P. A. Morel. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 23:983–987, 2005.
Ahrens, E. T., and J. Zhong. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 26:860–871, 2013.
Bhirde, A., J. Xie, M. Swierczewska, and X. Chen. Nanoparticles for cell labeling. Nanoscale 3:142–153, 2011.
Bible, E., F. Dell’Acqua, B. Solanky, A. Balducci, P. M. Crapo, S. F. Badylak, E. T. Ahrens, and M. Modo. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by 19F- and diffusion-MRI. Biomaterials 33:2858–2871, 2012.
Boehm-Sturm, P., L. Mengler, S. Wecker, M. Hoehn, and T. Kallur. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS ONE 6:e29040–e29049, 2011.
Bonetto, F., M. Srinivas, A. Heerschap, R. Mailliard, E. T. Ahrens, C. G. Figdor, and I. J. M. de Vries. A novel 19F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int. J. Cancer 129:365–373, 2010.
Bulte, J., J. W. Bulte, T. Douglas, T. Douglas, B. Witwer, B. Witwer, S. C. Zhang, E. Strable, E. Strable, B. K. Lewis, B. K. Lewis, H. Zywicke, H. Zywicke, B. Miller, B. Miller, P. van Gelderen, B. M. Moskowitz, B. M. Moskowitz, I. D. Duncan, and J. A. Frank. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 19:1141–1147, 2001.
Bulte, J. W. M., S. C. Zhang, P. van Gelderen, V. Herynek, E. K. Jordan, I. D. Duncan, and J. A. Frank. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc. Natl. Acad. Sci. 96:15256–15261, 1999.
Cromer Berman, S. M., K. C. J. Wang, I. Orukari, A. Levchenko, J. W. M. Bulte, and P. Walczak. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn. Reson. Med. 69:255–262, 2012.
De Feo, D., A. Merlini, C. Laterza, and G. Martino. Neural stem cell transplantation in central nervous system disorders. Curr. Opin. Neurol. 25:322–333, 2012.
Gilad, A. A., P. Walczak, M. T. McMahon, H. B. Na, J. H. Lee, K. An, T. Hyeon, P. C. M. van Zijl, and J. W. M. Bulte. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn. Reson. Med. 60:1–7, 2008.
Granot, D., M. K. Nkansah, M. F. Bennewitz, K. S. Tang, E. A. Markakis, and E. M. Shapiro. Clinically viable magnetic poly(lactide-co-glycolide) particles for MRI-based cell tracking. Magn. Reson. Med. 71:1238–1250, 2014.
Granot, D., D. Scheinost, E. A. Markakis, X. Papademetris, and E. M. Shapiro. Serial monitoring of endogenous neuroblast migration by cellular MRI. NeuroImage 57:817–824, 2011.
Gulaka, P. K., U. Rastogi, M. A. McKay, X. Wang, R. P. Mason, and V. D. Kodibagkar. Hexamethyldisiloxane-based nanoprobes for 1H MRI oximetry. NMR Biomed. 24:1226–1234, 2011.
Guzman, R., N. Uchida, T. M. Bliss, D. He, K. K. Christopherson, D. Stellwagen, A. Capela, J. Greve, R. C. Malenka, M. E. Moseley, T. D. Palmer, and G. K. Steinberg. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc. Natl. Acad. Sci. 104:10211–10216, 2007.
Janjic, J. M., and E. T. Ahrens. Fluorine-containing nanoemulsions for MRI cell tracking. WIREs Nanomed. Nanobiotechnol. 1:492–501, 2009.
Kodibagkar, V. D., W. Cui, M. E. Merritt, and R. P. Mason. Novel 1H NMR approach to quantitative tissue oximetry using hexamethyldisiloxane. Magn. Reson. Med. 55:743–748, 2006.
Kodibagkar, V. D., X. Wang, and R. P. Mason. Physical principles of quantitative nuclear magnetic resonance oximetry. Front. Biosci. J. Virtual Library 13:1371–1384, 2008.
Kodibagkar, V. D., X. Wang, J. Pacheco-Torres, P. Gulaka, and R. P. Mason. Proton imaging of siloxanes to map tissue oxygenation levels (PISTOL): a tool for quantitative tissue oximetry. NMR Biomed. 21:899–907, 2008.
Kraitchman, D. L., and J. W. M. Bulte. In vivo imaging of stem cells and Beta cells using direct cell labeling and reporter gene methods. Arterioscler. Thromb. Vasc. Biol. 29:1025–1030, 2009.
Laistler, E., M. Poirier-Quinot, S. A. Lambert, R.-M. Dubuisson, O. M. Girard, E. Moser, L. Darrasse, and J.-C. Ginefri. In vivo MR imaging of the human skin at subnanoliter resolution using a superconducting surface coil at 1.5 tesla. J. Magn. Reson. Imaging 41:496–504, 2013.
Lustig, M., D. Donoho, and J. M. Pauly. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 58:1182–1195, 2007.
Menon, J. U., P. K. Gulaka, M. A. McKay, S. Geethanath, L. Liu, and V. D. Kodibagkar. Dual-modality, dual-functional nanoprobes for cellular and molecular imaging. Theranostics 2:1199–1207, 2013.
Nabuurs, R. J. A., I. Hegeman, R. Natté, S. G. van Duinen, M. A. van Buchem, L. van der Weerd, and A. G. Webb. High-field MRI of single histological slices using an inductively coupled, self-resonant microcoil: application to ex vivo samples of patients with Alzheimer’s disease. NMR Biomed. 24:351–357, 2011.
Ramos-Gómez, M., E. G. Seiz, and A. Martínez-Serrano. Optimization of the magnetic labeling of human neural stem cells and MRI visualization in the hemiparkinsonian rat brain. J. Nanobiotechnol. 13:20, 2015.
Riess, P., C. Zhang, K. E. Saatman, H. L. Laurer, L. G. Longhi, R. Raghupathi, P. M. Lenzlinger, J. Lifshitz, J. Boockvar, and E. Neugebauer. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 51:1043–1054, 2002.
Ruiz-Cabello, J., P. Walczak, D. A. Kedziorek, V. P. Chacko, A. H. Schmieder, S. A. Wickline, G. M. Lanza, and J. W. M. Bulte. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn. Reson. Med. 60:1506–1511, 2008.
Srinivas, M., E. H. J. G. Aarntzen, J. W. M. Bulte, W. J. Oyen, A. Heerschap, I. J. M. de Vries, and C. G. Figdor. Imaging of cellular therapies. Adv. Drug Deliv. Rev. 62:1080–1093, 2010.
Srivastava, A. K., D. K. Kadayakkara, A. Bar-Shir, A. A. Gilad, M. T. McMahon, and J. W. M. Bulte. Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis. Models Mech. 8:323–336, 2015.
Vidya Shankar, R. and V. D. Kodibagkar. A rapid Look-Locker imaging sequence for quantitative tissue oximetry. In: Proceedings of the SPIE 9417, Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging, 94170F. doi:10.1117/12.2084009, 2015.
Wang, Z. J. Improving SNR of RF coils using composite coil elements. NMR Biomed. 22:952–959, 2009.
Zhong, J., P. H. Mills, T. K. Hitchens, and E. T. Ahrens. Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn. Reson. Med. 69:1683–1690, 2012.
Zhong, J., M. Sakaki, H. Okada, and E. T. Ahrens. In vivo intracellular oxygen dynamics in murine brain glioma and immunotherapeutic response of cytotoxic T cells observed by fluorine-19 magnetic resonance imaging. PLoS ONE 8:e59479-7, 2013.
Acknowledgments
The authors would like to acknowledge David Menn, Arizona State University, and Qingwei Liu, Barrow Neurological Institute, for technical assistance. These studies were supported by a Rising Stars in Engineering seed grant from College of Engineering, ASU (VDK and SES) and NIH 1DP2HD084067 (SES).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Agata Exner oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10439_2015_1514_MOESM1_ESM.tif
Flow cytometry scatter and gating of labeled NSPCs stained with calcein AM. Based on gating, indicated by black outline, NPSCs labeled at 1 μL/104 cells (B) did not exhibit an increase in the dead (gated) population compared to unlabeled NPSCs (A). At 5, 10 and 50 μL/104 cells labeling concentration, the dead population increased to 11.7%, 19.9% and 35.4% respectively (C-E). (TIFF 20116 kb)
10439_2015_1514_MOESM2_ESM.tif
NPSC radial migration on poly-L-lysine (PLL) and laminin out to 6 days. Minimal radial migration is observed on PLL at days 0, 3 and 6 for both unlabeled (A-C) and labeled (D-F) NPSCs. However, increased radial migration is observed on laminin at days 3 and 6 compared to day 0 and to PLL controls for both unlabeled (G-I) and labeled (J-L) NPSCs. Positive rhodamine B staining is indicative of labeling (D-F, J-L). Scale bar is 150 μm. (TIFF 63681 kb)
10439_2015_1514_MOESM3_ESM.tif
R1 versus pO2 calibration curves for PDMS nanoemulsion. R1 values of tubes with nanoemulsions equilibrated at 0%, 10%, and 21% atm oxygen (0, 76 and 160 torr respectively) and sealed show the expected linear dependence on pO2. Fitting yielded following calibration constants: intercept A’ = 0.235 ± 0.006 s-1 and slope B’ = (1.30 ± 0.08)X10-3 (s torr)-1, (R2 >0.99) at 23 ºC and A’ = 0.207 ± 0.001 s-1 and B’ = 1.25 ± 0.01)X10-3 (s torr)-1, (R2 >0.99) at 33.5 ºC. (TIFF 30081 kb)
Rights and permissions
About this article
Cite this article
Addington, C.P., Cusick, A., Shankar, R.V. et al. Siloxane Nanoprobes for Labeling and Dual Modality Functional Imaging of Neural Stem Cells. Ann Biomed Eng 44, 816–827 (2016). https://doi.org/10.1007/s10439-015-1514-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1514-1