Abstract
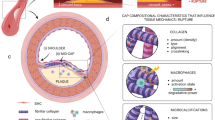
This review examines changing perspectives on the biomechanics of vulnerable plaque rupture over the past 25 years from the first finite element analyses (FEA) showing that the presence of a lipid pool significantly increases the local tissue stress in the atheroma cap to the latest imaging and 3D FEA studies revealing numerous microcalcifications in the cap proper and a new paradigm for cap rupture. The first part of the review summarizes studies describing the role of the fibrous cap thickness, tissue properties, and lesion geometry as main determinants of the risk of rupture. Advantages and limitations of current imaging technologies for assessment of vulnerable plaques are also discussed. However, the basic paradoxes as to why ruptures frequently did not coincide with location of PCS and why caps >65 μm thickness could rupture at tissue stresses significantly below the 300 kPa critical threshold still remained unresolved. The second part of the review describes recent studies in the role of microcalcifications, their origin, shape, and clustering in explaining these unresolved issues including the actual mechanism of rupture due to the explosive growth of tiny voids (cavitation) in local regions of high stress concentration between closely spaced microinclusions oriented along their tensile axis.









Similar content being viewed by others
References
Aikawa, E., et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 116(24):2841–2850, 2007.
Akyildiz, A. C., et al. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed. Eng. Online 10:25, 2011.
Barrett, S. R., M. P. Sutcliffe, S. Howarth, Z. Y. Li, and J. H. Gillard. Experimental measurement of the mechanical properties of carotid atherothrombotic plaque fibrous cap. J. Biomech. 42(11):1650–1655, 2009.
Bennett, M. R., G. I. Evan, and S. M. Schwartz. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Invest. 95(5):2266–2274, 1995.
Berliner, J. A., et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91(9):2488–2496, 1995.
Bluestein, D., et al. Influence of microcalcifications on vulnerable plaque mechanics using FSI modeling. J. Biomech. 41(5):1111–1118, 2008.
Bobryshev, Y. V., M. C. Killingsworth, R. S. Lord, and A. J. Grabs. Matrix vesicles in the fibrous cap of atherosclerotic plaque: possible contribution to plaque rupture. J. Cell Mol. Med. 12(5B):2073–2082, 2008.
Born, G. V. R., and P. D. Richardson. Mechanical properties of human atherosclerotic lesions. In: Pathology of the Human Atherosclerotic Plaque, edited by S. Glagov, W. P. Newman, and S. Shaffer. Berlin: Springer, 1989.
Born, G. V., and P. D. Richardson. A classic collaboration: Michael Davies on plaque vulnerability. Atherosclerosis 220(2):593–597, 2012.
Burke, A. P., et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 336(18):1276–1282, 1997.
Burke, A. P., et al. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 281(10):921–926, 1999.
Burleigh, M. C., et al. Collagen types I and III, collagen content, GAGs and mechanical strength of human atherosclerotic plaque caps: span-wise variations. Atherosclerosis 96(1):71–81, 1992.
Cheng, G. C., H. M. Loree, R. D. Kamm, M. C. Fishbein, and R. T. Lee. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 87(4):1179–1187, 1993.
Choi, B. J., et al. Comparison of 64-slice multidetector computed tomography with spectral analysis of intravascular ultrasound backscatter signals for characterizations of noncalcified coronary arterial plaques. Am. J. Cardiol. 102(8):988–993, 2008.
Choudhury, R. P., V. Fuster, J. J. Badimon, E. A. Fisher, and Z. A. Fayad. MRI and characterization of atherosclerotic plaque: emerging applications and molecular imaging. Arterioscler. Thromb. Vasc. Biol. 22(7):1065–1074, 2002.
Claes, E., et al. Mechanical properties of human coronary arteries. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010:3792–3795, 2010.
Davies, M. J., P. D. Richardson, N. Woolf, D. R. Katz, and J. Mann. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 69(5):377–381, 1993.
Davies, M. J., and T. Thomas. The pathological basis and microanatomy of occlusive thrombus formation in human coronary arteries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 294(1072):225–229, 1981.
Davies, M. J., and A. C. Thomas. Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 53(4):363–373, 1985.
de Korte, C. L., E. I. Cespedes, A. F. van der Steen, G. Pasterkamp, and N. Bom. Intravascular ultrasound elastography: assessment and imaging of elastic properties of diseased arteries and vulnerable plaque. Eur. J. Ultrasound 7(3):219–224, 1998.
Demer, L. L. Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int. J. Epidemiol. 31(4):737–741, 2002.
Ebenstein, D. M., D. Coughlin, J. Chapman, C. Li, and L. A. Pruitt. Nanomechanical properties of calcification, fibrous tissue, and hematoma from atherosclerotic plaques. J. Biomed. Mater. Res. A 91(4):1028–1037, 2009.
Ebenstein, D. M., et al. Assessing structure–property relations of diseased tissues using nanoindentation and FTIR. In: Advanced Biomaterials: Characterization, Tissue Engineering, and Complexity, edited by S. Moss. Boston, MA: Materials Research Society, 2002, pp. 47–52.
Ehara, S., et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110(22):3424–3429, 2004.
Finet, G., J. Ohayon, and G. Rioufol. Biomechanical interaction between cap thickness, lipid core composition and blood pressure in vulnerable coronary plaque: impact on stability or instability. Coron. Artery Dis. 15(1):13–20, 2004.
Fitzpatrick, L. A., A. Severson, W. D. Edwards, and R. T. Ingram. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J. Clin. Invest. 94(4):1597–1604, 1994.
Gent, A. N. Detachment of an elastic matrix from a rigid spherical inclusion. J. Mater. Sci. 15(11):2884–2888, 1980.
Gent, A. N., and B. Park. Failure processes in elastomers at or near a rigid spherical inclusion. J. Mater. Sci. 19(6):1947–1956, 1984.
Goodier, J. N. Concentration of stress around spherical and cylindrical inclusion and flaws. Trans. ASME 55:39–44, 1933.
Holzapfel, G. A., G. Sommer, C. T. Gasser, and P. Regitnig. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am. J. Physiol. Heart Circ. Physiol. 289(5):H2048–2058, 2005.
Holzapfel, G. A., G. Sommer, and P. Regitnig. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J. Biomech. Eng. 126(5):657–665, 2004.
Hoshino, T., et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am. J. Physiol. Heart Circ. Physiol. 297(2):H802–H810, 2009.
Hsu, H. H., and N. P. Camacho. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis 143(2):353–362, 1999.
Huang, H., et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103(8):1051–1056, 2001.
Isner, J. M., M. Kearney, S. Bortman, and J. Passeri. Apoptosis in human atherosclerosis and restenosis. Circulation 91(11):2703–2711, 1995.
Kelly-Arnold, A., et al. A revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc. Nat. Acad. Sci. U.S.A. 110(26):10741–10746, 2013.
Knollmann, F., et al. Quantification of atherosclerotic coronary plaque components by submillimeter computed tomography. Int. J. Cardiovasc. Imaging 24(3):301–310, 2008.
Kolodgie, F. D., et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 349(24):2316–2325, 2003.
Kopp, A. F., et al. Non-invasive characterisation of coronary lesion morphology and composition by multislice CT: first results in comparison with intracoronary ultrasound. Eur. Radiol. 11(9):1607–1611, 2001.
Kural, M. H., et al. Planar biaxial characterization of diseased human coronary and carotid arteries for computational modeling. J. Biomech. 45(5):790–798, 2012.
Larose, E., et al. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation 112(15):2324–2331, 2005.
Larose, E., et al. Improved characterization of atherosclerotic plaques by gadolinium contrast during intravascular magnetic resonance imaging of human arteries. Atherosclerosis 196(2):919–925, 2008.
Lawlor, M. G., M. R. O’Donnell, B. M. O’Connell, and M. T. Walsh. Experimental determination of circumferential properties of fresh carotid artery plaques. J. Biomech. 44(9):1709–1715, 2011.
Leach, J. R., et al. Carotid atheroma rupture observed in vivo and FSI-predicted stress distribution based on pre-rupture imaging. Ann. Biomed. Eng. 38(8):2748–2765, 2010.
Lee, R. T., A. J. Grodzinsky, E. H. Frank, R. D. Kamm, and F. J. Schoen. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation 83(5):1764–1770, 1991.
Lee, R. T., et al. Prediction of mechanical properties of human atherosclerotic tissue by high-frequency intravascular ultrasound imaging. An in vitro study. Arterioscler. Thromb. 12(1):1–5, 1992.
Lendon, C. L., A. D. Briggs, G. V. R. Born, M. C. Burleigh, and M. J. Davies. Mechanical testing of connective-tissue in the search for determinants of atherosclerotic plaque cap rupture. Biochem. Soc. Trans. 16(6):1032–1033, 1988.
Lendon, C. L., M. J. Davies, G. V. Born, and P. D. Richardson. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 87(1):87–90, 1991.
Lendon, C. L., M. J. Davies, P. D. Richardson, and G. V. R. Born. Testing of small connective-tissue specimens for the determination of the mechanical-behavior of atherosclerotic plaques. J. Biomed. Eng. 15(1):27–33, 1993.
Loree, H. M., A. J. Grodzinsky, S. Y. Park, L. J. Gibson, and R. T. Lee. Static circumferential tangential modulus of human atherosclerotic tissue. J. Biomech. 27(2):195–204, 1994.
Loree, H. M., R. D. Kamm, R. G. Stringfellow, and R. T. Lee. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ. Res. 71(4):850–858, 1992.
Maehara, A., et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J. Am. Coll. Cardiol. 40(5):904–910, 2002.
Maher, E., et al. Tensile and compressive properties of fresh human carotid atherosclerotic plaques. J. Biomech. 42(16):2760–2767, 2009.
Maldonado, N., A. Kelly-Arnold, L. Cardoso, and S. Weinbaum. The explosive growth of small voids in vulnerable cap rupture; cavitation and interfacial debonding. J. Biomech. 46(2):396–401, 2013.
Maldonado, N., et al. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: potential implications for plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 303(5):H619–H628, 2012.
Moreno, P. R., et al. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 105(8):923–927, 2002.
Motoyama, S., et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 50(4):319–326, 2007.
Nasu, K., et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J. Am. Coll. Cardiol. 47(12):2405–2412, 2006.
New, S. E. P., and E. Aikawa. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ. Res. 108(11):1381–1391, 2011.
Ohayon, J., G. Finet, F. Treyve, G. Rioufol, and O. Dubreuil. A three-dimensional finite element analysis of stress distribution in a coronary atherosclerotic plaque: in-vivo prediction of plaque rupture location. Biomech. Appl. Comput. Assist. Surg. 661:225–241, 2005.
Ohayon, J., et al. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 293(3):H1987–H1996, 2007.
Ohayon, J., et al. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 295(2):H717–H727, 2008.
Patwari, P., et al. Assessment of coronary plaque with optical coherence tomography and high-frequency ultrasound. Am. J. Cardiol. 85(5):641–644, 2000.
Potkin, B. N., et al. Coronary artery imaging with intravascular high-frequency ultrasound. Circulation 81(5):1575–1585, 1990.
Rambhia, S. H., et al. Microcalcifications increase coronary vulnerable plaque rupture potential: a patient-based micro-CT fluid–structure interaction study. Ann. Biomed. Eng. 40(7):1443–1454, 2012.
Richardson, P. D., M. J. Davies, and G. V. Born. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 2(8669):941–944, 1989.
Rodriguez-Granillo, G. A., et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J. Am. Coll. Cardiol. 46(11):2038–2042, 2005.
Russell, 3rd, R. R., and B. L. Zaret. Nuclear cardiology: present and future. Curr. Probl. Cardiol. 31(9):557–629, 2006.
Schaar, J. A., et al. Characterizing vulnerable plaque features with intravascular elastography. Circulation 108(21):2636–2641, 2003.
Sinusas, A. J. Molecular imaging in nuclear cardiology: translating research concepts into clinical applications. Q. J. Nucl. Med. Mol. Imaging 54(2):230–240, 2010.
Sommer, G., P. Regitnig, L. Koltringer, and G. A. Holzapfel. Biaxial mechanical properties of intact and layer-dissected human carotid arteries at physiological and supraphysiological loadings. Am. J. Physiol. Heart Circ. Physiol. 298(3):H898–912, 2010.
Stary, H. C. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am. J. Cardiol. 88(2A):16e–19e, 2001.
Stary, H. C. Atlas of Atherosclerosis: Progression and Regression (2nd ed.). The Encyclopedia of Visual Medicine Series. Boca Raton: The Parthenon Publishing Group, CRC Press, 144 pp., 2003.
Strauss, H. W., R. K. Grewal, and N. Pandit-Taskar. Molecular imaging in nuclear cardiology. Semin. Nucl. Med. 34(1):47–55, 2004.
Tanaka, A., et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation 118(23):2368–2373, 2008.
Tang, D., C. Yang, S. Kobayashi, and D. N. Ku. Effect of a lipid pool on stress/strain distributions in stenotic arteries: 3-D fluid–structure interactions (FSI) models. J. Biomech. Eng. 126(3):363–370, 2004.
Tang, D., et al. Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann. Biomed. Eng. 33(12):1789–1801, 2005.
Tarbell, J. M. Shear stress and the endothelial transport barrier. Cardiovasc. Res. 87(2):320–330, 2010.
Teng, Z., D. Tang, J. Zheng, P. K. Woodard, and A. H. Hoffman. An experimental study on the ultimate strength of the adventitia and media of human atherosclerotic carotid arteries in circumferential and axial directions. J. Biomech. 42(15):2535–2539, 2009.
Teng, Z., et al. 3D critical plaque wall stress is a better predictor of carotid plaque rupture sites than flow shear stress: an in vivo MRI-based 3D FSI study. J. Biomech. Eng. 132(3):031007, 2010.
Tsimikas, S., and P. X. Shaw. Non-invasive imaging of vulnerable plaques by molecular targeting of oxidized LDL with tagged oxidation-specific antibodies. J. Cell. Biochem. Suppl. 39:138–146, 2002.
Vengrenyuk, Y., L. Cardoso, and S. Weinbaum. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps. Mol. Cell. Biomech. 5(1):37–47, 2008.
Vengrenyuk, Y., T. J. Kaplan, L. Cardoso, G. J. Randolph, and S. Weinbaum. Computational stress analysis of atherosclerotic plaques in ApoE knockout mice. Ann. Biomed. Eng. 38(3):738–747, 2010.
Vengrenyuk, Y., et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Nat. Acad. Sci. U.S.A. 103(40):14678–14683, 2006.
Virmani, R., A. P. Burke, F. D. Kolodgie, and A. Farb. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J. Interv. Cardiol. 16(3):267–272, 2003.
Virmani, R., J. Narula, M. Leon, and J. T. E. Willerson. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management. Malden, MA: Blackwell, 2007.
Wenk, J. F. Numerical modeling of stress in stenotic arteries with microcalcifications: a parameter sensitivity study. J. Biomech. Eng. 133(1):014503, 2011.
Yabushita, H., et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 106(13):1640–1645, 2002.
Yang, F., et al. Segmentation of wall and plaque in in vitro vascular MR images. Int. J. Cardiovasc. Imaging 19(5):419–428, 2003.
Acknowledgments
This research has been supported by NIH ARRA grant RCI HL101151 to SW, AG034198, NSF MRI 0723027, 1229449, and PSC CUNY award to LC.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Robert Nerem oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Cardoso, L., Weinbaum, S. Changing Views of the Biomechanics of Vulnerable Plaque Rupture: A Review. Ann Biomed Eng 42, 415–431 (2014). https://doi.org/10.1007/s10439-013-0855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0855-x