Abstract
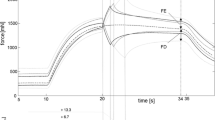
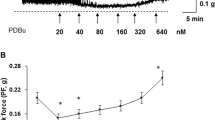
The length–tension (L–T) relationships in rabbit detrusor smooth muscle (DSM) are similar to those in vascular and airway smooth muscles and exhibit short-term length adaptation characterized by L–T curves that shift along the length axis as a function of activation and strain history. In contrast to skeletal muscle, the length–active tension (L–T a) curve for rabbit DSM strips does not have a unique peak tension value with a single ascending and descending limb. Instead, DSM can exhibit multiple ascending and descending limbs, and repeated KCl-induced contractions at a particular muscle length on an ascending or descending limb display increasingly greater tension. In the present study, mouse bladder strips with and without urothelium exhibited KCl-induced and carbachol-induced length adaptation, and the pressure–volume relationship in mouse whole bladder displayed short-term volume adaptation. Finally, prostaglandin-E2-induced low-level rhythmic contraction produced length adaptation in rabbit DSM strips. A likely role of length adaptation during bladder filling is to prepare DSM cells to contract efficiently over a broad range of volumes. Mammalian bladders exhibit spontaneous rhythmic contraction (SRC) during the filling phase and SRC is elevated in humans with overactive bladder (OAB). The present data identify a potential physiological role for SRC in bladder adaptation and motivate the investigation of a potential link between short-term volume adaptation and OAB with impaired contractility.






Similar content being viewed by others
Abbreviations
- 0-Ca:
-
Nominally Ca2+-free solution consisting of PSS without CaCl2
- APS:
-
Adjustable preload stiffness
- CCh:
-
Carbachol
- DSM:
-
Detrusor smooth muscle
- KPSS:
-
PSS modified to include 110 mM KCl substituted isosmotically for NaCl
- L ref :
-
Reference length corresponding to T ref
- L s :
-
Slack length
- L–T :
-
Length–tension
- L–T a :
-
Length–active tension
- L–T p :
-
Length–preload tension
- PGE2 :
-
Prostaglandin-E2
- PSS:
-
Physiological salt solution
- P ref :
-
Reference pressure, a peak active pressure value on a P–V curve
- P–V :
-
Pressure–volume
- RC:
-
Rhythmic contraction
- SRC:
-
Spontaneous rhythmic contraction
- T a :
-
Active tension
- T p :
-
Preload tension
- T ref :
-
Reference tension, a peak T a value on a L–T a curve
- T t :
-
Total tension
- Uro:
-
Urothelium
- V ref :
-
Reference volume corresponding to P ref
References
Abrams, P., and A. J. Wein. Introduction: overactive bladder and its treatment. Urology 55:1–2, 2000.
Almasri, A. M., P. H. Ratz, H. Bhatia, A. P. Klausner, and J. E. Speich. Rhythmic contraction generates adjustable passive stiffness in rabbit detrusor. J. Appl. Physiol. 108(3):544–553, 2010.
Almasri, A. M., P. H. Ratz, and J. E. Speich. Length adaptation of the passive-to-active tension ratio in rabbit detrusor. Ann. Biomed. Eng. 38(8):2594–2605, 2010.
Bai, T. R., J. H. Bates, V. Brusasco, B. Camoretti-Mercado, P. Chitano, L. H. Deng, M. Dowell, B. Fabry, L. E. Ford, J. J. Fredberg, W. T. Gerthoffer, S. H. Gilbert, S. J. Gunst, C. M. Hai, A. J. Halayko, S. J. Hirst, A. L. James, L. J. Janssen, K. A. Jones, G. G. King, O. J. Lakser, R. K. Lambert, A. M. Lauzon, K. R. Lutchen, G. N. Maksym, R. A. Meiss, S. M. Mijailovich, H. W. Mitchell, R. W. Mitchell, W. Mitzner, T. M. Murphy, P. D. Paré, R. R. Schellenberg, C. Y. Seow, G. C. Sieck, P. G. Smith, A. V. Smolensky, J. Solway, N. L. Stephens, A. G. Stewart, D. D. Tang, and L. Wang. On the terminology for describing the length-force relationship and its changes in airway smooth muscle. J. Appl. Physiol. 97:2029–2034, 2004.
Bednarek, M. L., J. E. Speich, A. S. Miner, and P. H. Ratz. Active tension adaptation at a shortened arterial muscle length: inhibition by cytochalasin-D. Am. J. Physiol. Heart Circ. Physiol. 300(4):H1166–H1173, 2011.
Bosse, Y., L. Y. Chin, P. D. Pare, and C. Y. Seow. Adaptation of airway smooth muscle to basal tone: relevance to airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 40(1):13–18, 2009.
Bosse, Y., D. Solomon, L. Y. Chin, K. Lian, P. D. Pare, and C. Y. Seow. Increase in passive stiffness at reduced airway smooth muscle length: potential impact on airway responsiveness. Am. J. Physiol. Lung Cell. Mol. Physiol. 298(3):L277–L287, 2010.
Brown, S. M., L. M. Bentcheva-Petkova, L. Liu, K. L. Hristov, M. Chen, W. F. Kellett, A. L. Meredith, R. W. Aldrich, M. T. Nelson, and G. V. Petkov. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am. J. Physiol. Renal Physiol. 295(4):F1149–F1157, 2008.
Collins, C., A. P. Klausner, B. Herrick, H. P. Koo, A. S. Miner, S. C. Henderson, and P. H. Ratz. Potential for control of detrusor smooth muscle spontaneous rhythmic contraction by cyclooxygenase products released by interstitial cells of cajal. J. Cell Mol. Med. 13(9B):3236–3250, 2009.
Drake, M. J., I. J. Harvey, and J. I. Gillespie. Autonomous activity in the isolated guinea pig bladder. Exp. Physiol. 88(1):19–30, 2003.
Drake, M. J., I. J. Harvey, J. I. Gillespie, and W. A. Van Duyl. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 95(7):1002–1005, 2005.
Dulin, N. O., D. J. Fernandes, M. Dowell, S. Bellam, J. McConville, O. Lakser, R. Mitchell, B. Camoretti-Mercado, P. Kogut, and J. Solway. What evidence implicates airway smooth muscle in the cause of BHR? Clin. Rev. Allergy Immunol. 24(1):73–84, 2003.
Ford, L. E. Plasticity in airway smooth muscle: an update. Can. J. Physiol. Pharmacol. 83(10):841–850, 2005.
Ford, L. E., C. Y. Seow, and V. R. Pratusevich. Plasticity in smooth muscle, a hypothesis. Can. J. Physiol. Pharmacol. 72(11):1320–1324, 1994.
Geeves, M. A., R. Fedorov, and D. J. Manstein. Molecular mechanism of actomyosin-based motility. Cell. Mol. Life Sci. 62(13):1462–1477, 2005.
Gillespie, J. I. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int. 93(4):478–483, 2004.
Gillespie, J. I. Modulation of autonomous contractile activity in the isolated whole bladder of the guinea pig. BJU Int. 93(3):393–400, 2004.
Gordon, A. M., A. F. Huxley, and F. J. Julian. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184(1):170–192, 1966.
Granzier, H. L., and T. C. Irving. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68(3):1027–1044, 1995.
Gunst, S. J., R. A. Meiss, M. F. Wu, and M. Rowe. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am. J. Physiol. 268(5 Pt 1):C1267–C1276, 1995.
Gunst, S. J., D. D. Tang, and A. Opazo Saez. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir. Physiol. Neurobiol. 137(2–3):151–168, 2003.
Hai, C. M., and R. A. Murphy. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am. J. Physiol. 254(1 Pt 1):C99–C106, 1988.
Iorga, B., N. Adamek, and M. A. Geeves. The slow skeletal muscle isoform of myosin shows kinetic features common to smooth and non-muscle myosins. J. Biol. Chem. 282(6):3559–3570, 2007.
Jezior, J. R., J. D. Brady, D. I. Rosenstein, K. A. McCammon, A. S. Miner, and P. H. Ratz. Dependency of detrusor contractions on calcium sensitization and calcium entry through LOE-908-sensitive channels. Br. J. Pharmacol. 134:78–87, 2001.
Kanai, A., J. Roppolo, Y. Ikeda, I. Zabbarova, C. Tai, L. Birder, D. Griffiths, W. de Groat, and C. Fry. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am. J. Physiol. Renal Physiol. 292(3):F1065–F1072, 2007.
Klausner, A. P., C. M. Johnson, A. B. Stike, J. E. Speich, V. Sabarwal, A. S. Miner, M. Cleary, H. P. Koo, and P. H. Ratz. Prostaglandin E2 mediates spontaneous rhythmic contraction in rabbit detrusor muscle. Can. J. Urol. 18(2):5608–5614, 2011.
Kock, N. G., and R. Pompeius. Studies on the nature of the rhythmic activity of the human bladder. Invest. Urol. 1:253–261, 1963.
Mills, I. W., J. E. Greenland, G. McMurray, R. McCoy, K. M. Ho, J. G. Noble, and A. F. Brading. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J. Urol. 163(2):646–651, 2000.
Nyitrai, M., and M. A. Geeves. Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359(1452):1867–1877, 2004.
Poley, R. N., C. R. Dosier, J. E. Speich, A. S. Miner, and P. H. Ratz. Stimulated calcium entry and constitutive RhoA kinase activity cause stretch-induced detrusor contraction. Eur. J. Pharmacol. 599:137–145, 2008.
Pratusevich, V. R., C. Y. Seow, and L. E. Ford. Plasticity in canine airway smooth muscle. J. Gen. Physiol. 105(1):73–94, 1995.
Ratz, P. H., and J. E. Speich. Evidence that actomyosin cross bridges contribute to “passive” tension in detrusor smooth muscle. Am. J. Physiol. Renal Physiol. 298(6):F1424–F1435, 2010.
Resnick, N. M., and S. V. Yalla. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA 257(22):3076–3081, 1987.
Ruegg, C., C. Veigel, J. E. Molloy, S. Schmitz, J. C. Sparrow, and R. H. Fink. Molecular motors: force and movement generated by single myosin II molecules. News Physiol. Sci. 17:213–218, 2002.
Seow, C. Y. Response of arterial smooth muscle to length perturbation. J. Appl. Physiol. 89(5):2065–2072, 2000.
Seow, C. Y., V. R. Pratusevich, and L. E. Ford. Series-to-parallel transition in the filament lattice of airway smooth muscle. J. Appl. Physiol. 89(3):869–876, 2000.
Sherrington, C. S. Notes on the arrangement of some motor fibres in the lumbo-sacral plexus. J. Physiol. 13(6):621–772, 1892.
Smolensky, A. V., and L. E. Ford. The extensive length-force relationship of porcine airway smooth muscle. J. Appl. Physiol. 102(5):1906–1911, 2007.
Speich, J. E., A. M. Almasri, H. Bhatia, A. P. Klausner, and P. H. Ratz. Adaptation of the length-active tension relationship in rabbit detrusor. Am. J. Physiol. Renal Physiol. 297(4):F1119–F1128, 2009.
Speich, J. E., L. Borgsmiller, C. Call, R. Mohr, and P. H. Ratz. ROK-induced cross-link formation stiffens passive muscle: reversible strain-induced stress softening in rabbit detrusor. Am. J. Physiol. Cell Physiol. 289(1):C12–C21, 2005.
Speich, J. E., C. Dosier, L. Borgsmiller, K. Quintero, H. P. Koo, and P. H. Ratz. Adjustable passive length-tension curve in rabbit detrusor smooth muscle. J. Appl. Physiol. 102(5):1746–1755, 2007.
Speich, J. E., K. Quintero, C. Dosier, L. Borgsmiller, H. P. Koo, and P. H. Ratz. A mechanical model for adjustable passive stiffness in rabbit detrusor. J. Appl. Physiol. 101(4):1189–1198, 2006.
Speich, J. E., J. B. Southern, S. Henderson, C. W. Wilson, A. P. Klausner, and P. H. Ratz. Adjustable passive stiffness in mouse bladder: regulated by Rho kinase and elevated following partial bladder outlet obstruction. Am. J. Physiol. Renal Physiol. 302(8):F967–F976, 2012.
Uvelius, B. Isometric and isotonic length-tension relations and variations in longitudinal smooth muscle from rabbit urinary bladder. Acta Physiol. Scand. 97(1):1–12, 1976.
Veigel, C., J. E. Molloy, S. Schmitz, and J. Kendrick-Jones. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat. Cell Biol. 5(11):980–986, 2003.
Whittaker, M., E. M. Wilson-Kubalek, J. E. Smith, L. Faust, R. A. Milligan, and H. L. Sweeney. A 35-A movement of smooth muscle myosin on ADP release. Nature 378(6558):748–751, 1995.
Wu, X., K. G. Morgan, C. J. Jones, R. M. Tribe, and M. J. Taggart. Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodelling. J. Cell Mol. Med. 12(4):1360–1373, 2008.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Amy S. Miner. This study was supported by a grant from the Edwin Beer Research Program in Urology and Urology Related Fields from the New York Academy of Medicine (to J.E.S.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan Jockenhoevel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Speich, J.E., Wilson, C.W., Almasri, A.M. et al. Carbachol-Induced Volume Adaptation in Mouse Bladder and Length Adaptation via Rhythmic Contraction in Rabbit Detrusor. Ann Biomed Eng 40, 2266–2276 (2012). https://doi.org/10.1007/s10439-012-0590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0590-8