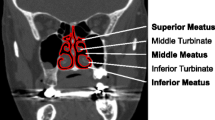
Abstract
Patient-specific studies of physiological flows rely on anatomically realistic or idealized models. Objective comparison of datasets or the relation of specific to idealized geometries has largely been performed in an ad hoc manner. Here, two rational procedures (based respectively on Fourier descriptors and medial axis (MA) transforms) are presented; each provides a compact representation of a complex anatomical region, specifically the nasal airways. The techniques are extended to furnish average geometries. These retain a sensible anatomical form, facilitating the identification of a specific anatomy as a set of weighted perturbations about the average. Both representations enable a rapid translation of the surface description into a virtual model for computation of airflow, enabling future work to comprehensively investigate the relation between anatomic form and flow-associated function, for the airways or for other complex biological conduits. The methodology based on MA transforms is shown to allow flexible geometric modeling, as illustrated by a local alteration in airway patency. Computational simulations of steady inspiratory flow are used to explore the relation between the flow in individual vs. averaged anatomical geometries. Results show characteristic flow measures of the averaged geometries to be within the range obtained from the original three subjects, irrespective of averaging procedure. However the effective regularization of anatomic form resulting from the shape averaging was found to significantly reduce trans-nasal pressure loss and the mean shear stress in the cavity. It is suggested that this may have implications in attempts to relate model geometries and flow patterns that are broadly representative.














Similar content being viewed by others
References
Anthony, T. R. Contribution of facial feature dimensions and velocity parameters on particle inhalability. Ann. Occup. Hyg. 54(6)710–725, 2010.
Aujay, G., F. Hétroy, F. Lazarus, and C. Depraz. Harmonic skeleton for realistic character animation. In: Proceedings of the 2007 ACM SIGGRAPH/Eurographics Symposium on Computer Animation, San Diego, CA, 02–04 August 2007, pp. 151–160.
Baroody, F. M. Functional anatomy of the upper airway in humans. In: Toxicology of the Nose and Upper Airways, edited by J. B. Morris and D. J. Shusterman. New York: Informa Healthcare, 2010, pp. 18–44.
Besl, P., and N. McKay. A method for registration of 3-D shapes. IEEE Trans. Pattern Anal. Mach. Intell. 14(2):239–256, 1992.
Bloomenthal, J. An implicit surface polygonizer. In: Graphics Gems IV. San Diego: Academic Press Professional, Inc., pp. 324–349, 1994.
Blum, H. A transformation for extracting new descriptors of shape. In: Models for the Perception of Speech and Visual Form. Cambridge: MIT Press, 1967, pp. 362–380.
Carr, J. C., W. R. Fright, and R. K. Beatson. Surface interpolation with radial basis functions for medical imaging. IEEE Trans. Med. Imaging. 16(1):96–107, 1997.
Churchill, S. E., L. L. Shacklford, J. N. Georgi, and M. T. Black. Morphological variation and airflow dynamics in the human nose. Am. J. Hum. Biol. 16:625–638, 2004.
Doorly, D. J., D. J. Taylor, A. M. Gambaruto, R. C. Schroter, and N. Tolley. Nasal architecture: form and flow. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 366(1879):3225–3246, 2008.
Doorly, D. J., D. J. Taylor, and R. C. Schroter. Mechanics of airflow in the human nasal airways. Respir. Physiol. Neurobiol. 163(1–3):100–110, 2008.
D. Eberly, R. Gardner, B. Morse, S. Pizer, and C. Scharlach. Ridges for image analysis. J. Math. Imaging Vis. 4(4):353–373, 1994.
Eccles, R. A role for the nasal cycle in respiratory defence. Eur. Respir. J. 9(2):371–376, 1996.
Fletcher, P. T., C. Lu, and S. Joshi. Statistics of shape via principal component analysis on lie groups. In: Proceedings of IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR), Vol. 1, pp. 95–101, 2003.
Gambaruto, A. M. Form and Flow in Anatomical Conduits: Bypass Graft and Nasal Cavity. PhD thesis, Aeronautical Engineering, Imperial College, University of London, 2007.
Gambaruto, A. M., D. J. Doorly, and T. Yamaguchi. Wall shear stress and near-wall convective transport: comparisons with vascular remodelling in a peripheral graft anastomosis. J. Comput. Phys. 229(14):5339–5356, 2010.
Gambaruto, A. M., A. Moura, and A. Sequeira. Topological flow structures and stir mixing for steady flow in a peripheral bypass graft with uncertainty. Int. J. Numer. Methods Biomed. Eng. 26(7): 926–953, 2010.
Gambaruto, A. M., J. Peiro, D. J. Doorly, and A. G. Radaelli. Reconstruction of shape and its effect on flow in arterial conduits. Int. J. Numer. Methods Fluids. 57(5):495–517, 2008.
Gambaruto, A. M., D. J. Taylor, and D. J. Doorly. Modelling nasal airflow using a Fourier descriptor representation of geometry. Int. J. Numer. Methods Fluids. 59(11):1259–1283, 2009.
Giordana, S., S. J. Sherwin, J. Peiró, D. J. Doorly, Y. Papaharilaou, C. G. Caro, N. Watkins, N. Cheshire, M. Jackson, C. Bicknall, and V. Zervas. Automated classification of peripheral distal by-pass geometries reconstructed from medical data. J. Biomech. 38(1):47–62, 2005.
Illum, L. Nasal drug delivery: possibilities, problems and solutions. J. Control. Release. 87:187–198, 2003.
Kappagantu, R. V., and B. F. Feenny. Part I: Dynamical Characterization of a Frictionally Excited Beam. Nonlinear Dyn. 22(4):317–333, 2000.
Kimmel, R., D. Shaked, and N. Kiryati. Skeletonization via distance maps and level sets. Comput. Vis. Mach. Underst. 62(3):382–391, 1995.
Kleven, M., M. C. Melaaen, M. Reimers, J. S. Rotnes, L. Aurdal, and P. G. Djupesland. Using computational fluid dynamics (CFD) to improve the bi-directional nasal drug delivery concept. Food Bioprod. Process. 83(C2):107–117, 2005.
Lang, J. Clinical Anatomy of the Nose, Nasal Cavity, and Paranasal Sinuses. New York: Thieme-Stratton Corp., 1989.
Liu, Y., M. R. Johnson, E. A. Matida, S. Kherani, and J. Marsan. Creation of a standardized geometry of the human nasal cavity. J. Appl. Physiol. 106(3):784–795, 2009.
Millán, R. D., L. Dempere-Marco, J. M. Pozo, J. R. Cebral, and A. F. Frangi. Morphological characterization of intracranial aneurysms using 3-D moment invariants. IEEE Trans. Med. Imaging. 26(9):1270–1282, 2007.
Mygind, N., and D. R. Anatomy. Physiology and function of the nasal cavities in health and disease. Adv. Drug Deliv. Rev. 29:3–11, 1998.
Piccinelli, M., A. Veneziani, D. A. Steinman, A. Remuzzi, and L. Antiga. A framework for geometric analysis of vascular structures: application to cerebral aneurysms. IEEE Trans. Med. Imaging. 28(8):1141–1155, 2009.
Rennie, C. E.., K. A. Gouder, D. J. Taylor, N. S. Tolley, R. C. Schroter, and D. J. Doorly. Nasal inspiratory flow: at rest and sniffing. Int. Forum Allergy Rhinol. 1(2):128–135, 2011.
Rusinkiewicz, S., and M. Levoy. Efficient variants of the ICP Algorithm. In: Proceedings of Third international Conference on 3D Digital Imaging and Modeling (3DIM), Quebec City, Canada, 28 May–1 June 2001.
Schreck, S., K. J. Sullivan, C. M. Ho, and H. K. Chang. Correlations between flow resistance and geometry in a model of the human nose. J. Appl. Physiol. 75(4):1767–1775, 1993.
Schroeter, J. D., J. S. Kimbell, and B. Asgharian. Analysis of particle deposition in the turbinate and olfactory regions using a human nasal computational fluid dynamic model. J. Aerosol Med. 19:301–313, 2006.
Shaked, D., and A. M. Bruckstein. Pruning medial axes. Comput. Vis. Image Underst. 69(2):156–169, 1998.
Shen, L., J. Ford, F. Makedon, and A. Saykin. A surface-based approach for classification of 3D neuroanatomic structures. Intell. Data Anal. 8(6/2004):519–542, 2004.
Tam, R., and W. Heidrich. Shape Simplification Based on the Medial Axis Transform. In: 14th IEEE Visualization 2003 (VIS 2003), Seattle, Washington, 22–24 October 2003.
Taylor, D. J., D. J. Doorly, and R. C. Schroter. Inflow boundary profile prescription for numerical simulation of nasal airflow. J. R. Soc. Interface. 7(44):515–527, 2010.
Tobor, I., P. Reuter, and C. Schlick. Efficient Reconstruction of Large Scattered Geometric Datasets using the Partition of Unity and Radial Basis Functions. In: Research Report RR-1301-03, Laboratoire Bordelais de Recherche en Informatique, Université Bordeaux, 2003.
Wang, S., and M. K. Chung. Parametrization and classification of closed anatomical curves. In: Technical Report, 1113, Department of Statistics, University of Wisconsin, 2005.
Wang, Y., X. Gu, P. M. Thompson, and S. Yau. 3D Harmonic Mapping and Tetrahedral Meshing of Brain Imaging Data. In: Proceedings of Medical Imaging Computing and Computer Assisted Intervention (MICCAI), St. Malo, France, 26–30 September 2004.
Weeks, A. R., Jr. Fundamentals of electronic image processing. SPIE/IEEE Ser Image Sci. Eng. 333–359, 452–470, 1996.
Wolf, M., S. Naftali, R. C. Schroter, and D. Elad. Air-conditioning characteristics of the human nose. J. Laryngol. Otol. 118:87–92, 2004.
Zahn, C. T., and R. Z. Roskies. Fourier descriptors for plane closed curves. IEEE Trans. Comput. c-21(3):269–281, 1972.
Zakaria, H., A. M. Robertson, and C. W. Kerber. A parametric model for studies of flow in arterial bifurcations. Ann. Biomed. Eng. 36(9):1515–1530, 2008.
Acknowledgments
The authors are grateful for the medical image datasets and assistance provided by the ENT department, St. Mary’s Hospital, Paddington, London and for the support of the BBSRC, through grant ref. BB/E02344/1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor John H. Linehan oversaw the review of this article.
Appendix
Appendix
Implicit Function
In brief, the interpolating surface is defined as the zero-level iso-surface of an implicit function \(f(\mathbf{x}).\) Setting \(f(\mathbf{x})=0\) on sampled points of the cross-section stack, defines the on-surface constraints. A gradient is formed in the implicit function by introducing further constraints at a constant close distance normal to the curve, known as off-surface constraints, with \(f(\mathbf{x})<-\lambda\) inside the curves and \(f(\mathbf{x})>\lambda\) outside the curves, where λ is a constant. Interpolation of these constraints leads to the solution of the unknown coefficients \(\mathbf{c}\) from a linear system given by \(f(\mathbf{x}_{i})= \sum^{n}_{j=1}\mathbf{c}_{j}\phi(\mathbf{x}_{i}-\mathbf{x}_{j}),\) for \(i=1,\ldots,n,\) where n is the number of constraints. Here the weighting function is given by the cubic RBF (RBF), hence \(\phi(\mathbf{x}_{i}-\mathbf{x}_{j})= |\mathbf{x}_{i}-\mathbf{x}_{j}|^{3},\) where \(|\cdot|\) denotes the Euclidean norm, \(\mathbf{x}_{i}\) are the position vectors the function is evaluated at and \(\mathbf{x}_{j}\) are the interpolation constraints, for \(\mathbf{x}_{j}=(x_{j},y_{j},z_{j});\quad j=1,\ldots,n.\) This choice of RBF is such that it minimises curvature variation.
The zero-level iso-surface of the implicit function that defines the virtual model surfaces is extracted using the marching tetrahedra approach5 with linear interpolation to give an initial triangulation, which was then projected onto the true zero-level iso-surface.
To reduce the computational time in the implicit function formulation as well as the marching tetrahedra method, a partition-of-unity approach15,37 is used. This divides the global domain of interest into smaller overlapping sub-domains where the problem can be solved locally. The local solutions are combined together by using weighting functions that act as smooth blending functions to obtain the global solution.
Medial Axis
The approach used in this work is the following: a two-dimensional closed curve is defined with edge, E. A medial axis S is then defined as the set of points whose closest distance, D, from the nearest boundary is locally maximum.22 The local value of D, denoted by d ij , is obtained for a uniform sampling grid centered at the center of mass of E and made up of \(n_{\zeta_{1}} \times n_{\zeta_{2}}\) points p ij , where \(i=1,\ldots,n_{\zeta_{1}},j=1,\ldots,n_{\zeta_{2}},\zeta_{1}\) and \(\zeta_{2}\) are orthogonal axes of the sampling grid, and \(\zeta_{1}\) is chosen as the major axis of E. The grid is typically formed by choosing \(n_{\zeta_{1}} = 1000\) and maintaining the spacing yields \(n_{\zeta_{2}} \approx 400\) for the middle section of the nasal cavity. If an interrogation point p ij on the grid is inside E then d ij > 0 and if outside d ij < 0. Hence, a value of d ij = 0 signifies that p ij lies on E. If the points on the grid are given an out-of-plane distance proportional to d ij , the object would resemble a hilly landscape where S would be the resultant connected crests (or ridgeline).11 The local maximum is extracted using a 3 × 3 local mask and results in a cluster of points that are then connected, ensuring that different branches are identified. Finally interpolation is performed to yield smooth fitting curves that are the medial axes and represent the supporting frame of E.
This procedure is sensitive to the resolution of the evaluation grid, generating spurious or insignificant branches if overly fine. This sensitivity arises if the piecewise linear segments that form E are comparable or larger than the grid scale, and hence at the junction of each segment a MA is formed. High grid sampling is however needed to accurately capture the medial axes. An automatic pruning method is adopted such that each branch is checked to identify spurious terminal branches by calculating its gradient \(g= \frac{\text{length\;of\;branch}}{\text{branch calibre at root}}.\) A criterion for the classification of a branch as spurious can be set based on the value of g. In the present case, a limit of g ≤ 1.1 was found to be robust in determining whether branches should be removed, although the choice is somewhat arbitrary and different values may be required for other datasets. Other pruning methods are described in Weeks et al.,40 Tam and Heidrich,35 and Aujay et al.33 which may be beneficial for other applications. For example an alternative approach is to consider branch lengths, such that if shorter than a given value they can be considered as small features and removed without significantly altering the object.
The pruning acts as a filter, reducing the level of detail by incrementally removing features of the smallest magnitude. This will therefore also progressively reduce the surface area and volume of the object. A small uniform inflation of the medial axes calibre is hence required, to account for the volume lost due to the pruning procedure. This inflation does not effectively change the surface area of the geometry boundary due to the high aspect ratio of the sections. Details of the surface areas and volumes of the cases studied are detailed in Tables 1 and 2.
Fourier Descriptor
Let us consider a closed curve γ(l) that can be expressed as a signal g(l), where l is the perimeter-length at a certain location and 0 ≤ l ≤ L, such that the signal has period L. The discrete Fourier expansion of g(l) is formulated by sampling at m equally spaced points to yield \(g(k);k=0, \ldots,m-1.\) The discrete Fourier transform (DFT) is given by
where \(i=\sqrt{-1}.\) The real and complex components of c(n) are the FDs, denoted as R(n) and I(n), respectively. The amplitude, or energy, of the nth mode in the series expansion is given by \(\sqrt{R(n)^2+I(n)^2}.\) To reconstruct g(k) the inverse discrete Fourier transform (IDFT) is used, which is given by
Signals of the closed curve may be formulated by considering the change in angular direction,42 however in this work the location in fixed Cartesian coordinate frame38 is used. The advantage in providing a signal in terms of the angular direction variation along the curve is that only one signal is required, while if using a Cartesian coordinate approach an orthogonal signal for each axis is required. In this work the Cartesian coordinate approach is used since it allows a closed contour to be reconstructed after filtering, averaging or other signal manipulations that is not possible otherwise. A brief description of this approach is now given.
A curve can be described as the change of location coordinates as a function of l, hence implementable directly in \({\mathbb{R}^{3}}\) even when the planar cross-sections are not aligned to the Cartesian axes. The signal that describes the curve is given by g(l) = (x(l) − x(0), y(l) − y(0), z(l) − z(0)), providing an orthogonal signal for each axis that allows for independent signal modification if desired. It is important to note that since the signals are periodic, a reconstructed closed curve in ensured.
Rights and permissions
About this article
Cite this article
Gambaruto, A.M., Taylor, D.J. & Doorly, D.J. Decomposition and Description of the Nasal Cavity Form. Ann Biomed Eng 40, 1142–1159 (2012). https://doi.org/10.1007/s10439-011-0485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0485-0