Abstract
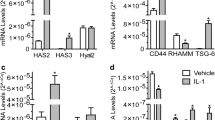
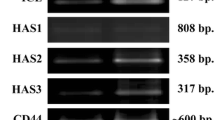
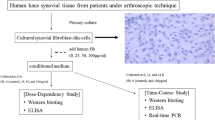
Hyaluronan (HA) plays a crucial role in the lubricating and buffering properties of synovial fluid. The purpose of this study was to examine the effects of interleukin (IL)-1β on HA degradation in cultured synovial membrane cells. The rabbit synovial membrane cell line HIG-82 was cultured with and without IL-1β. The amounts of HA of varying molecular weights in the medium were analyzed using high-performance liquid chromatography, the mRNA levels of HA synthase (HAS) and hyaluronidase (HYAL) were analyzed by means of real-time PCR, and HYAL activity was analyzed by HA zymography. The amounts of HA with a molecular weight lower than 300 kDa, and between 300 and 1900 kDa, in the culture medium of HIG-82 cells were significantly higher in the presence of IL-1β. However, the amount of HA with a molecular weight greater than 1900 kDa was significantly lower in the presence of IL-1β. Both HAS2 and HAS3 mRNA levels were upregulated by treatment with IL-1β. So, too, were the levels of HYAL1 and HYAL2 mRNA, which resulted in enhanced HYAL activity. However, HYAL activity was inhibited by transfection of HYAL2-siRNA. Our results suggest that IL-1β is a crucial factor in the fragmentation of HA in inflammatory joints.





Similar content being viewed by others
References
Bjelle, A., T. Andersson, and K. Granath. Molecular weight distribution of hyaluronic acid of human synovial fluid in rheumatic diseases. Scand. J. Rheumatol. 12(2):133–138, 1983.
Csoka, A. B., G. I. Frost, and R. Stern. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 20(8):499–508, 2001.
Flannery, C. R., C. B. Little, C. E. Hughes, and B. Caterson. Expression and activity of articular cartilage hyaluronidases. Biochem. Biophys. Res. Commun. 251(3):824–829, 1998.
Frost, G. I., T. B. Csoka, T. Wong, and R. Stern. Purification, cloning, and expression of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 236(1):10–15, 1997.
Frost, G. I., and R. Stern. A microtiter-based assay for hyaluronidase activity not requiring specialized reagents. Anal. Biochem. 251(2):263–269, 1997.
Guntenhoner, M. W., M. A. Pogrel, and R. Stern. A substrate-gel assay for hyaluronidase activity. Matrix 12(5):388–396, 1992.
Harada, H., and M. Takahashi. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 282(8):5597–5607, 2007.
Itano, N., T. Sawai, M. Yoshida, P. Lenas, Y. Yamada, M. Imagawa, T. Shinomura, M. Hamaguchi, Y. Yoshida, Y. Ohnuki, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Immunol. 274(35):25085–25092, 1999.
Konttinen, Y. T., H. Saari, and D. C. Nordstrom. Effect of interleukin-1 on hyaluronate synthesis by synovial fibroblastic cells. Clin. Rheumatol. 10(2):151–154, 1991.
Kreil, G. Hyaluronidases—a group of neglected enzymes. Protein Sci. 4(9):1666–1669, 1995.
Laurent, T. C., and J. R. Fraser. Hyaluronan. Faseb J. 6(7):2397–2404, 1992.
Lepperdinger, G., B. Strobl, and G. Kreil. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J. Biol. Chem. 273(35):22466–22470, 1998.
McNeil, J. D., O. W. Wiebkin, W. H. Betts, and L. G. Cleland. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann. Rheum. Dis. 44(11):780–789, 1985.
Meyer, F. A., I. Yaron, and M. Yaron. Synergistic, additive, and antagonistic effects of interleukin-1 beta, tumor necrosis factor alpha, and gamma-interferon on prostaglandin E, hyaluronic acid, and collagenase production by cultured synovial fibroblasts. Arthritis Rheum. 33(10):1518–1525, 1990.
Nagaya, H., T. Ymagata, S. Ymagata, K. Iyoda, H. Ito, Y. Hasegawa, and H. Iwata. Examination of synovial fluid and serum hyaluronidase activity as a joint marker in rheumatoid arthritis and osteoarthritis patients (by zymography). Ann. Rheum. Dis. 58(3):186–188, 1999.
Oertli, B., B. Beck-Schimmer, X. Fan, and R. P. Wuthrich. Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kappa B and activating protein-1. J. Immunol. 161(7):3431–3437, 1998.
Orkin, R. W., and B. P. Toole. Isolation and characterization of hyaluronidase from cultures of chick embryo skin- and muscle-derived fibroblasts. J. Biol. Chem. 255(3):1036–1042, 1980.
Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad Sci. USA 98(8):4443–4448, 2001.
Sampson, P. M., C. L. Rochester, B. Freundlich, and J. A. Elias. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production. Evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. J. Clin. Invest. 90(4):1492–1503, 1992.
Schlaak, J. F., I. Pfers, K. H. Meyer Zum Buschenfelde, and E. Marker-Hermann. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 14(2):155–162, 1996.
Smith, J. B., M. H. Bocchieri, L. Sherbin-Allen, M. Borofsky, and J. L. Abruzzo. Occurrence of interleukin-1 in human synovial fluid: detection by RIA, bioassay and presence of bioassay-inhibiting factors. Rheumatol. Int. 9(2):53–58, 1989.
Stair-Nawy, S., A. B. Csoka, and R. Stern. Hyaluronidase expression in human skin fibroblasts. Biochem. Biophys. Res. Commun. 266(1):268–273, 1999.
Stern, R. Hyaluronan catabolism: a new metabolic pathway. Eur. J. Cell Biol. 83(7):317–325, 2004.
Stern, R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol. Biol. (Paris) 53(7):372–382, 2005; (Epub 2005 Jan 19).
Strobl, B., C. Wechselberger, D. R. Beier, and G. Lepperdinger. Structural organization and chromosomal localization of Hyal2, a gene encoding a lysosomal hyaluronidase. Genomics 53(2):214–219, 1998.
Suzuki, T., N. Segami, M. Nishimura, and T. Nojima. Co-expression of interleukin-1beta and tumor necrosis factor alpha in synovial tissues and synovial fluids of temporomandibular joint with internal derangement: comparison with histological grading of synovial inflammation. J. Oral Pathol. Med. 31(9):549–557, 2002.
Tanimoto, K., S. Ohno, K. Fujimoto, K. Honda, C. Ijuin, N. Tanaka, T. Doi, M. Nakahara, and K. Tanne. Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect. Tissue Res. 42(3):187–195, 2001.
Tanimoto, K., A. Suzuki, S. Ohno, K. Honda, N. Tanaka, T. Doi, K. Yoneno, M. Ohno-Nakahara, Y. Nakatani, M. Ueki, et al. Effects of TGF-beta on hyaluronan anabolism in fibroblasts derived from the synovial membrane of the rabbit temporomandibular joint. J. Dent. Res. 83(1):40–44, 2004.
Yoshida, M., S. Sai, K. Marumo, T. Tanaka, N. Itano, K. Kimata, and K. Fujii. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res. Ther. 6(6):514–520, 2004.
Acknowledgment
This research was supported by a Grant-in-Aid (No. 19659540) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ceng Dong oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Tanimoto, K., Yanagida, T., Tanne, Y. et al. Modulation of Hyaluronan Fragmentation by Interleukin-1 Beta in Synovial Membrane Cells. Ann Biomed Eng 38, 1618–1625 (2010). https://doi.org/10.1007/s10439-010-9927-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9927-3