Abstract
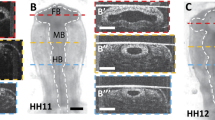
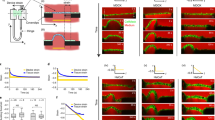
The mechano-sensitive responses of the heart and brain were examined in the chick embryo during Hamburger and Hamilton stages 10–12. During these early stages of development, cells in these structures are organized into epithelia. Isolated hearts and brains were compressed by controlled amounts of surface tension (ST) at the surface of the sample, and microindentation was used to measure tissue stiffness following several hours of culture. The response of both organs was qualitatively similar, as they stiffened under reduced loading. With increased loading, however, the brain softened while heart stiffness was similar to controls. In the brain, changes in nuclear shape and morphology correlated with these responses, as nuclei became more elliptical with decreased loading and rounder with increased loading. Exposure to the myosin inhibitor blebbistatin indicated that these changes in stiffness and nuclear shape are likely caused by altered cytoskeletal contraction. Computational modeling suggests that this behavior tends to return peak tissue stress back toward the levels it has in the intact heart and brain. These results suggest that developing cardiac and neural epithelia respond similarly to changes in applied loads by altering contractility in ways that tend to restore the original mechanical stress state. Hence, this study supports the view that stress-based mechanical feedback plays a role in regulating epithelial development.








Similar content being viewed by others
References
Beloussov, L. V. The Dynamic Architecture of a Developing Organism: An Interdisciplinary Approach to the Development of Organisms. Dordrecht, The Netherlands: Kluwer, 1998.
Beloussov, L. V. Mechanically based generative laws of morphogenesis. Phys. Biol. 5:15009, 2008.
Beloussov, L. V., and V. I. Grabovsky. Morphomechanics: goals, basic experiments and models. Int. J. Dev. Biol. 50:81–92, 2006.
Butler, J. K. An Experimental Analysis of Cardiac Loop Formation in the Chick. M.S. thesis, University of Texas, 1952.
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292:H1209–H1224, 2007.
Chowdhury, F., S. Na, D. Li, Y. C. Poh, T. S. Tanaka, F. Wang, and N. Wang. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 9:82–88, 2010.
Clark, E. B., N. Hu, P. Frommelt, G. K. Vandekieft, J. L. Dummett, and R. J. Tomanek. Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am. J. Physiol. 257:H55–H61, 1989.
Dahl, K. N., A. J. Ribeiro, and J. Lammerding. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 102:1307–1318, 2008.
Desmond, M. E., and A. G. Jacobson. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev. Biol. 57:188–198, 1977.
Desmond, M. E., M. L. Levitan, and A. R. Haas. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 285:737–747, 2005.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Fernandez-Gonzalez, R., and J. A. Zallen. Cell mechanics and feedback regulation of actomyosin networks. Sci. Signal. 2:pe78, 2009.
Filas, B. A., I. R. Efimov, and L. A. Taber. Optical coherence tomography as a tool for measuring morphogenetic deformation of the looping heart. Anat. Rec. 290:1057–1068, 2007.
Filas, B. A., A. K. Knutsen, P. V. Bayly, and L. A. Taber. A new method for measuring deformation of folding surfaces during morphogenesis. J. Biomech. Eng. 130:061010, 2008.
Fujimoto, J. G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 21:1361–1367, 2003.
Galbraith, C. G., R. Skalak, and S. Chien. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskeleton 40:317–330, 1998.
Goodrum, G. R., and A. G. Jacobson. Cephalic flexure formation in the chick embryo. J. Exp. Zool. 216:399–408, 1981.
Guilak, F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J. Biomech. 28:1529–1541, 1995.
Gutzman, J. H., E. G. Graeden, L. A. Lowery, H. S. Holley, and H. Sive. Formation of the zebrafish midbrain–hindbrain boundary constriction requires laminin-dependent basal constriction. Mech. Dev. 125:974–983, 2008.
Hamburger, V., and H. L. Hamilton. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92, 1951.
Humphrey, J. D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50:53–78, 2008.
Jaalouk, D. E., and J. Lammerding. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10:63–73, 2009.
Jenkins, M. W., F. Rothenberg, D. Roy, V. P. Nikolski, Z. Hu, M. Watanabe, D. L. Wilson, I. R. Efimov, and A. M. Rollins. 4D embryonic cardiography using gated optical coherence tomography. Opt. Express 14:736–748, 2006.
Jurisicova, A., S. Varmuza, and R. F. Casper. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 2:93–98, 1996.
Kassab, G. S., and Navia, J. A. Biomechanical considerations in the design of graft: the homeostasis hypothesis. Annu. Rev. Biomed. Eng. 8:499–535, 2006.
Kornikova, E. S., T. G. Troshina, S. V. Kremnyov, and L. V. Beloussov. Neuro-mesodermal patterns in artificially deformed embryonic explants: a role for mechano-geometry in tissue differentiation. Dev. Dyn. 239:885–896, 2010.
Krieg, M., Y. Arboleda-Estudillo, P. H. Puech, J. Kafer, F. Graner, D. J. Muller, and C. P. Heisenberg. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10:429–436, 2008.
Maniotis, A. J., C. S. Chen, and D. E. Ingber. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA 94:849–854, 1997.
Manner, J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 259:248–262, 2000.
Mizutani, T., H. Haga, and K. Kawabata. Cellular stiffness response to external deformation: tensional homeostasis in a single fibroblast. Cell Motil. Cytoskeleton 59:242–248, 2004.
Munro, E. M., and G. M. Odell. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development 129:13–24, 2002.
Nelson, C. M., R. P. Jean, J. L. Tan, W. F. Liu, N. J. Sniadecki, A. A. Spector, and C. S. Chen. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl Acad. Sci. USA 102:11594–11599, 2005.
Nerurkar, N. L., A. Ramasubramanian, and L. A. Taber. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Dev. Dyn. 235:1822–1829, 2006.
Pajerowski, J. D., K. N. Dahl, F. L. Zhong, P. J. Sammak, and D. E. Discher. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104:15619–15624, 2007.
Pouille, P. A., P. Ahmadi, A. C. Brunet, and E. Farge. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2:ra16, 2009.
Ramasubramanian, A., N. L. Nerurkar, K. H. Achtien, B. A. Filas, D. A. Voronov, and L. A. Taber. On modeling morphogenesis of the looping heart following mechanical perturbations. J. Biomech. Eng. 130:061018, 2008.
Reinhart-King, C. A., M. Dembo, and D. A. Hammer. Cell–cell mechanical communication through compliant substrates. Biophys. J. 95:6044–6051, 2008.
Remond, M. C., J. A. Fee, E. L. Elson, and L. A. Taber. Myosin-based contraction is not necessary for cardiac c-looping in the chick embryo. Anat. Embryol. (Berl.) 211:443–454, 2006.
Saez, A., M. Ghibaudo, A. Buguin, P. Silberzan, and B. Ladoux. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl Acad. Sci. USA 104:8281–8286, 2007.
Taber, L. A. Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48:487–545, 1995.
Taber, L. A. Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 50:323–332, 2006.
Taber, L. A. Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech. Model. Mechanobiol. 7:427–441, 2008.
Taber, L. A. Towards a unified theory for morphomechanics. Philos. Trans. A Math. Phys. Eng. Sci. 367:3555–3583, 2009.
Trepat, X., L. Deng, S. S. An, D. Navajas, D. J. Tschumperlin, W. T. Gerthoffer, J. P. Butler, and J. J. Fredberg. Universal physical responses to stretch in the living cell. Nature 447:592–595, 2007.
Voronov, D. A., and L. A. Taber. Cardiac looping in experimental conditions: the effects of extraembryonic forces. Dev. Dyn. 224:413–421, 2002.
Wang, N., J. D. Tytell, and D. E. Ingber. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10:75–82, 2009.
Wozniak, M. A., and C. S. Chen. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10:34–43, 2009.
Xu, G., P. S. Kemp, J. A. Hwu, A. M. Beagley, P. V. Bayly, and L. A. Taber. Opening angles and material properties of the early embryonic chick brain. J. Biomech. Eng.-Trans. ASME 132:071013, 2010.
Zamir, E. A., V. Srinivasan, R. Perucchio, and L. A. Taber. Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 31:1327–1336, 2003.
Acknowledgments
We thank Igor Efimov for access to his OCT system, as well as Anjul Davis at Thorlabs for access to the integrated microscope + OCT system that aided our isolated sample imaging. We also thank Dmitry Voronov and Dylan McCreedy for sectioning and staining advice, in addition to Shelly Sakiyama-Elbert for microscope access. Finally, we acknowledge Guy Genin for helpful insight into our stress analysis of the brain. This study was supported by NSF grant DMS-0540701 (LAT), NIH grant R01 GM075200 (LAT), and fellowships for BAF from NIH T90 DA022871 and the Mallinckrodt Institute of Radiology, Washington University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Filas, B.A., Bayly, P.V. & Taber, L.A. Mechanical Stress as a Regulator of Cytoskeletal Contractility and Nuclear Shape in Embryonic Epithelia. Ann Biomed Eng 39, 443–454 (2011). https://doi.org/10.1007/s10439-010-0171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0171-7