Abstract
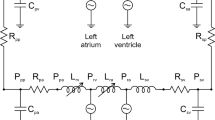
Aortic flow and pressure result from the interactions between the heart and arterial system. In this work, we considered these interactions by utilizing a lumped parameter heart model as an inflow boundary condition for three-dimensional finite element simulations of aortic blood flow and vessel wall dynamics. The ventricular pressure–volume behavior of the lumped parameter heart model is approximated using a time varying elastance function scaled from a normalized elastance function. When the aortic valve is open, the coupled multidomain method is used to strongly couple the lumped parameter heart model and three-dimensional arterial models and compute ventricular volume, ventricular pressure, aortic flow, and aortic pressure. The shape of the velocity profiles of the inlet boundary and the outlet boundaries that experience retrograde flow are constrained to achieve a robust algorithm. When the aortic valve is closed, the inflow boundary condition is switched to a zero velocity Dirichlet condition. With this method, we obtain physiologically realistic aortic flow and pressure waveforms. We demonstrate this method in a patient-specific model of a normal human thoracic aorta under rest and exercise conditions and an aortic coarctation model under pre- and post-interventions.










Similar content being viewed by others
References
Asanoi, H., T. Kameyama, S. Ishizaka, T. Nozawa, and H. Inoue. Energetically optimal left ventricular pressure for the failing human heart. Circulation 93(1):67–63, 1996.
Brooks, A. N., and T. J. R. Hughes. Streamline upwind/Petrov-Galerkin formulations for convection dominated flows with particular emphasis on the incompressible Navier-Stokes equations. Comput. Methods Appl. Mech. Eng. 32:199–259, 1982.
Cebral, J. R., M. A. Castro, J. E. Burgess, R. S. Pergolizzi, M. J. Sheridan, and C. M. Putman. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am. J. Neuroradiol. 26(10):2550–2559, 2005.
Figueroa, C. A., I. E. Vignon-Clementel, K. E. Jansen, T. J. R. Hughes, and C. A. Taylor. A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput. Methods Appl. Mech. Eng. 195(41–43):5685–5706, 2006.
Formaggia, L., J. F. Gerbeau, F. Nobile, and A. Quarteroni. On the coupling of 3D and 1D Navier-Stokes equations for flow problems in compliant vessels. Comput. Methods Appl. Mech. Eng. 191(6–7):561–582, 2001.
Formaggia, L., D. Lamponi, M. Tuveri, and A. Veneziani. Numerical modeling of 1D arterial networks coupled with a lumped parameters description of the heart. Comput. Meth. Biomech. Biomed. Eng. 9(5):273–288, 2006.
Franca, L. P., and S. L. Frey. Stabilized finite element methods: II. The incompressible Navier-Stokes equations. Comput. Methods Appl. Mech. Eng. 99(2–3):209–233, 1992.
Heywood, J., R. Rannacher, and S. Turek. Artificial boundaries and flux and pressure conditions for the incompressible Navier-Stokes equations. Int. J. Numer. Methods Fluids 22(5):325–352, 1996.
Hope, S. A., D. B. Tay, I. T. Meredith, and J. D. Cameron. Waveform dispersion, not reflection, may be the major determinant of aortic pressure wave morphology. Am. J. Physiol. Heart Circ. Physiol. 289(6):H2497–H2502, 2005.
Hunter, P. J., A. J. Pullan, and B. H. Smaill. Modeling total heart function. Annu. Rev. Biomed. Eng. 5(1):147–177, 2003.
Kerckhoffs, R. C. P., M. L. Neal, Q. Gu, J. B. Bassingthwaighte, J. H. Omens, and A. D. McCulloch. Coupling of a 3D finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonic circulation. Ann. Biomed. Eng. 35(1):1–18, 2007.
Kim, H. J., C. A. Figueroa, T. J. R. Hughes, K. E. Jansen, and C. A. Taylor. Augmented lagrangian method for constraining the shape of velocity profiles at outlet boundaries for three-dimensional finite element simulations of blood flow. Comput. Methods Appl. Mech. Eng. (in press). doi:10.1016/j.cma.2009.02.012
Kirklin, J. W., and B. G. Barratt-Boyes. Cardiac Surgery: Morphology, Diagnostic Criteria, Natural History, Techniques, Results, and Indications, 2nd edition. New York: W.B. Saunders, 1993.
Laskey, W. K., H. G. Parker, V. A. Ferrari, W. G. Kussmaul, and A. Noordergraaf. Estimation of total systemic arterial compliance in humans. J. Appl. Physiol. 69(1):112–119, 1990.
Li, Z., and C. Kleinstreuer. Blood flow and structure interactions in a stented abdominal aortic aneurysm model. Med. Eng. Phys. 27(5):369–382, 2005.
Migliavacca, F., R. Balossino, G. Pennati, G. Dubini, T. H. Hsia, M. R. de Leval, and E. L. Bove. Multiscale modelling in biofluidynamics: application to reconstructive paediatric cardiac surgery. J. Biomech. 39(6):1010–1020, 2006.
Ottesen, J. T., M. S. Olufsen, and J. K. Larsen. Applied Mathematical Models in Human Physiology. SIAM Monographs on Mathematical Modeling and Computation. Philadelphia: SIAM, 2004.
Perktold, K., R. Peter, and M. Resch. Pulsatile non-Newtonian blood flow simulation through a bifurcation with an aneurysm. Biorheology 26(6):1011–1030, 1989.
Quarteroni, A., S. Ragni, and A. Veneziani. Coupling between lumped and distributed models for blood flow problems. Comput. Vis. Sci., 4(2):111–124, 2001.
Sahni, O., J. Muller, K. E. Jansen, M. S. Shephard, and C. A. Taylor. Efficient anisotropic adaptive discretization of the cardiovascular system. Comput. Methods Appl. Mech. Eng. 195(41–43):5634–5655, 2006.
Sainte-Marie, J., D. Chapelle, R. Cimrman, and M. Sorine. Modeling and estimation of the cardiac electromechanical activity. Comput. Struct. 84:1743–1759, 2006.
Seear, M., S. Webber, and J. Leblanc. Descending aortic blood flow velocity as a noninvasive measure of cardiac output in children. Pediatr. Cardiol. 15(4):178–183, 1994.
Segers, P., N. Stergiopulos, and N. Westerhof. Relation of effective arterial elastance to arterial system properties. Am. J. Physiol. Heart Circ. Physiol. 282(3):H1041–H1046, 2002.
Segers, P., N. Stergiopulos, N. Westerhof, P. Wouters, P. Kolh, and P. Verdonck. Systemic and pulmonary hemodynamics assessed with a lumped-parameter heart-arterial interaction model. J. Eng. Math. 47(3):185–199, 2003.
Senzaki, H., C. H. Chen, and D. A. Kass. Single-beat estimation of end-systolic pressure-volume relation in humans: a new method with the potential for noninvasive application. Circulation 94(10):2497–2506, 1996.
Soerensen, D. D., K. Pekkan, D. de Zelicourt, S. Sharma, K. Kanter, M. Fogel, and A. P. Yoganathan. Introduction of a new optimized total cavopulmonary connection. Ann. Thorac. Surg. 83(6):2182–2190, 2007.
Stergiopulos, N., P. Segers, and N. Westerhof. Use of pulse pressure method for estimating total arterial compliance in vivo. Am. J. Physiol. Heart Circ. Physiol. 276(2):H424–H428, 1999.
Stuhne, G. R., and D. A. Steinman. Finite-element modeling of the hemodynamics of stented aneurysms. J. Biomech. Eng. 126(3):382–387, 2004.
Suga, H., and K. Sagawa. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ. Res. 35(1):117–126, 1974.
Tang, B. T., C. P. Cheng, M. T. Draney, N. M. Wilson, P. S. Tsao, R. J. Herfkens, and C. A. Taylor. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am. J. Physiol. Heart Circ. Physiol. 291(2):H668–H676, 2006.
Taylor, C. A., and M. T. Draney. Experimental and computational methods in cardiovascular fluid mechanics. Annu. Rev. Fluid Mech. 36(1):197–231, 2004.
Taylor, C. A., M. T. Draney, J. P. Ku, D. Parker, B. N. Steele, K. Wang, and C. K. Zarins. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aid. Surg. 4(5):231–247, 1999.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Finite element modeling of blood flow in arteries. Comput. Methods Appl. Mech. Eng. 158(1–2):155–196, 1998.
Taylor, S. H., and K. W. Donald. Circulatory studies at rest and during exercise in coarctation of the aorta before and after operation. Br. Heart J. 22:117–139, 1960.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195(29–32):3776–3796, 2006.
Whiting, C. H., and K. E. Jansen. A stabilized finite element method for the incompressible Navier-Stokes equations using a hierarchical basis. Int. J. Numer. Methods Fluids 35(1):93–116, 2001.
Zamir, M., P. Sinclair, and T. H. Wonnacott. Relation between diameter and flow in major branches of the arch of the aorta. J. Biomech. 25(11):1303–1310, 1992.
Acknowledgments
Hyun Jin Kim was supported by a Stanford Graduate Fellowship. This material is based upon work supported by the National Science Foundation under Grant No. 0205741. The authors gratefully acknowledge Dr. Nathan M. Wilson for assistance with software development. The authors gratefully acknowledge Dr. Farzin Shakib for the use of his linear algebra package AcuSolveTM (http://www.acusim.com) and the support of Simmetrix, Inc for the use of the MeshSimTM (http://www.simmetrix.com) mesh generator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.J., Vignon-Clementel, I.E., Figueroa, C.A. et al. On Coupling a Lumped Parameter Heart Model and a Three-Dimensional Finite Element Aorta Model. Ann Biomed Eng 37, 2153–2169 (2009). https://doi.org/10.1007/s10439-009-9760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9760-8