Abstract
Left ventricular remodeling during the development of heart failure is a strong predictor of cardiovascular mortality. However, methods to objectively quantify remodeling-associated shape changes are not routinely available but may be possible with new computational anatomy tools. In this study, we analyzed and compared multi-detector computed tomographic (MDCT) images of ventricular shape at end-systole (ES) and end-diastole (ED) to determine whether regional structural characteristics could be identified and, as a proof of principle, whether differences in hearts of patients with anterior myocardial infarction (MI) and ischemic cardiomyopathy (ICM) could be distinguished from those with global nonischemic cardiomyopathy (NICM). MDCT images of hearts from 11 patients (5 with ICM) with ejection fractions (EF) < 35% were analyzed. An average ventricular shape model (template) was constructed for each cardiac phase by bringing heart shapes into correspondence using linear and nonlinear image matching algorithms. Next, transformation fields were computed between the template image and individual heart images in the population. Principal component analysis (PCA) method was used to quantify ventricular shape differences described by the transformation vector fields. Statistical analysis of PCA coefficients revealed significant ventricular shape differences at ED (p = 0.03) and ES (p = 0.03). For validation, a second set of 14 EF-matched patients (8 with ICM) were evaluated. The discrimination rule learned from the training data set was able to differentiate ICM from NICM patients (p = 0.008). Application of a novel shape analysis method to in vivo human cardiac images acquired on a clinical scanner is feasible and can quantify regional shape differences at end-systole in remodeled myopathic human myocardium. This approach may be useful in identifying differences in the remodeling process between ICM and NICM populations and possibly in differentiating the populations.








Similar content being viewed by others
References
Anderson, T. (1958) An Introduction to Multivariate Statistical Analysis. New York: Wiley.
Beg, M. F., M. I. Miller, A. Trouv’e and L. B. Younes (2005) Computing Large Deformation Metric Mappings via Geodesic Flows of Diffeomorphisms. International Journal of Computer Vision 61:139-157. doi:10.1023/B:VISI.0000043755.93987.aa
Douglas, P. S., R. Morrow, A. Ioli and N. Reichek 1989 Left ventricular shape, afterload and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 13:311-315.
Eichhorn, E. J. and M. R. Bristow 1996 Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation 94:2285-2296.
Felker, G. M., L. K. Shaw and C. M. O’Connor 2002 A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 39:210-218. doi:10.1016/S0735-1097(01)01738-7
Grenander, U. and M. I. Miller 1998 Computational anatomy: an emerging discipline. Q. Appl. Math. LVI:617–694.
Helm, P., M. F. Beg, M. I. Miller and R. L. Winslow 2005 Measuring and mapping cardiac fiber and laminar architecture using diffusion tensor MR imaging. Ann NY Acad Sci 1047:296-307. doi:10.1196/annals.1341.026
Helm, P. A., L. Younes, M. F. Beg, D. B. Ennis, C. Leclercq, O. P. Faris, E. McVeigh, D. Kass, M. I. Miller and R. L. Winslow 2006 Evidence of structural remodeling in the dyssynchronous failing heart. Circ Res 98:125-132. doi:10.1161/01.RES.0000199396.30688.eb
Hunt, S. A., D. W. Baker, M. H. Chin, M. P. Cinquegrani, A. M. Feldman, G. S. Francis, T. G. Ganiats, S. Goldstein, G. Gregoratos, M. L. Jessup, R. J. Noble, M. Packer, M. A. Silver, L. W. Stevenson, R. J. Gibbons, E. M. Antman, J. S. Alpert, D. P. Faxon, V. Fuster, G. Gregoratos, A. K. Jacobs, L. F. Hiratzka, R. O. Russell and S. C. Smith, Jr. 2001 ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Circulation 104:2996-3007. doi:10.1161/hc4901.102568
Jenkinson, M., P. Bannister, M. Brady and S. Smith 2002 Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825-841. doi:10.1016/S1053-8119(02)91132-8
Jolliffe, I. T. Principal Component Analysis. Springer, 2002, 502 pp.
Joshi, S., U. Grenander and M. j. g. Miller 1997 the geometry and shape of brain sub-manifolds. International Journal of Pattern Recognition and Artificial Intelligence: Special Issue on Processing of Magnetic Resonance Imaging 11:1317-1343. doi:10.1142/S0218001497000615
Jugdutt, B. I. 1990 Identification of patients prone to infarct expansion by the degree of regional shape distortion on an early two-dimensional echocardiogram after myocardial infarction. Clin Cardiol 13:28-40.
Komamura, K., R. P. Shannon, T. Ihara, Y. T. Shen, I. Mirsky, S. P. Bishop and S. F. Vatner 1993 Exhaustion of Frank-Starling mechanism in conscious dogs with heart failure. Am J Physiol Heart Circ Physiol 265:H1119-1131.
Lee, T. H., M. A. Hamilton, L. W. Stevenson, J. D. Moriguchi, G. C. Fqnarow, J. S. Child, H. Laks and J. A. Walden 1993 Impact of left ventricular cavity size on survival in advanced heart failure. The American Journal of Cardiology 72:672-676. doi:10.1016/0002-9149(93)90883-E
Meluzin, J., L. Spinarova, P. Hude, J. Krejci, L. Dusek, J. Vitovec and R. Panovsky 2005 Combined right ventricular systolic and diastolic dysfunction represents a strong determinant of poor prognosis in patients with symptomatic heart failure. Int J Cardiol 105:164-173. doi:10.1016/j.ijcard.2004.12.031
Miller, M., A. Banerjee, G. Christensen, S. Joshi, N. Khaneja, U. Grenander and L. Matejic 1997 Statistical methods in computational anatomy. Stat Methods Med Res 6:267-299. doi:10.1191/096228097673360480
Miller, M. I., G. E. Christensen, Y. Amit and U. Grenander 1993 Mathematical textbook of deformable neuroanatomies. Proc Natl Acad Sci U S A 90:11944-11948. doi:10.1073/pnas.90.24.11944
Miller, M. I., A. Trouve, and L. Younes. Geodesic shooting for computational anatomy. J. Math. Imag. Vision V24:209–228, 2006
Ota, T., C. E. Fleishman, M. Strub, G. Stetten, C. J. Ohazama, O. T. von Ramm and J. Kisslo 1999 Real-time, three-dimensional echocardiography: feasibility of dynamic right ventricular volume measurement with saline contrast. Am Heart J 137:958-966. doi:10.1016/S0002-8703(99)70422-9
Salm, L. P., J. D. Schuijf, A. de Roos, H. J. Lamb, H. W. Vliegen, J. W. Jukema, R. Joemai, E. E. van der Wall and J. J. Bax 2006 Global and regional left ventricular function assessment with 16-detector row CT: comparison with echocardiography and cardiovascular magnetic resonance. European Journal of Echocardiography 7:308-314. doi:10.1016/j.euje.2005.07.002
St John Sutton, M., D. Lee, J. L. Rouleau, S. Goldman, T. Plappert, E. Braunwald and M. A. Pfeffer 2003 Left Ventricular Remodeling and Ventricular Arrhythmias After Myocardial Infarction. Circulation 107:2577-2582. doi:10.1161/01.CIR.0000065226.24159.E9
Sugeng, L., L. Weinert, K. Thiele and R. M. Lang 2003 Real-time three-dimensional echocardiography using a novel matrix array transducer. Echocardiography 20:623-635. doi:10.1046/j.1540-8175.2003.t01-1-03031.x
Nichols, T. E., Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping 15:1–25. doi:10.1002/hbm.1058
Wang, L., S. C. Joshi, M. I. Miller and J. G. Csernansky 2001 Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage 14:531-545. doi:10.1006/nimg.2001.0830
Acknowledgments
This research was supported by Grants from the National Institute of Health (HL70894, HL52307, R24 HL085343), the American Heart Association (0725357U), and the Donald W. Reynolds Cardiovascular Clinical Research Center at The Johns Hopkins Hospital. The authors would like to thank Dr. Patrick Helm for his helpful technical comments. Dr. Weiss received research funds from Reynolds foundation and NIH (HL61912). Drs. Lardo, George, and Lima received research funds from Toshiba, Inc. Dr. Lardo received speaker’s honoraria from Toshiba, Inc. Dr. George received speaker’s bureau from Toshiba, Inc. and serves as a consultant for Medrad, Inc. Dr. Wu received research funds from General Electric Healthcare. The remaining authors report no conflicts.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
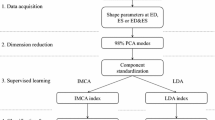
Methods of Heart Shape Analysis
Quantification of anatomical variation requires transformation of different members of an ensemble of anatomical structures (in this case, heart images obtained in ED and ES) into a common extrinsic coordinate system. In computational anatomy, structures are represented using a deformable template (a representative heart image, one for ES and one for ED) and anatomies (specific heart images) are generated using a set of diffeomorphic (smooth and invertible) transformations acting on the template.17 First, a 9 parameter affine intensity based registration algorithm minimizing a cost function based on mutual information was used for course alignment of anatomies with respect to rotations, translations, and scalings.10 Next, a non-rigid high dimensional intensity based transformation method (the Large-Deformation Diffeomorphic Metric Mapping (LDDMM) algorithm,2 described below) was used to match images at the voxel level. In this algorithm, optimal correspondences between images are estimated by computing geodesics (paths of minimal energy) in the shape space and are solutions to a system of partial differential equations. For each target heart a unique evolution (geodesic) path that deforms the template geometry onto the target is defined by an initial velocity vector field applied to the template geometry. Therefore, from the template and the initial momentum (a quantity derived from the initial velocity vector field), the entire geodesic path between template and target geometry may be reconstructed. This reconstruction process using the initial momentum is referred to as geodesic shooting.
The Large-Deformation Diffeomorphic Metric Mapping (LDDMM) Algorithm
Let Ω ⊆ R n (n = 3 for 3D space) be a bounded domain on which the image functions I: Ω→R d (with d = 1 for scalar intensity images such as CT scan) are defined. In this model, image function I defines an ensemble of observed anatomical imagery “I” which is an orbit under diffeomorphic transformations G with the law of composition \( \psi \cdot \varphi = \psi \circ \varphi . \) For any \(\varphi\) in the set of continuous smooth and invertible transformations and any image I, \( \varphi \cdot I = I \circ \varphi^{ - 1} \) defines an action of G on “I”. Given two images, I 0 and I 1, the task is to find a transformation \(\varphi\) that register these two images \( \left( {I_{1} = \varphi \cdot I_{0} = I_{0} \circ \varphi^{ - 1} } \right). \) This deformation is estimated as the end point of a time indexed flow (φ = ϕ 1) associated with a smooth, compactly supported velocity vector field \( v_{t} \in V,\,t \in [0,1]. \) These two entities are related by: \( \frac{d}{dt}\phi_{t}^{v} (x) = v_{t} \left( {\phi_{t}^{v} (x)} \right) \) where boundary points are defined as \( \phi_{0}^{v} (x) = Id(x) = x,\, \forall x \in \Upomega \) and \( \phi_{1}^{v} (x) = \varphi \in \Upomega . \) The optimal solution can be achieved by the integration of the above formula after computing the optimal velocity through this variational problem:
The diffeomorphism is ensured by enforcing sufficient smoothness on the space of allowable velocity vector fields V via defining a differential operator L of type \( L = ( - \alpha \Updelta + \gamma )^{\beta } I_{nxn} \) where β > 1.5 in 3D space such that \( \left\| f \right\|_{v} = \left\| {Lf} \right\|_{L^{2}} \) where \( \left\| . \right\|_{L^{2}} \) is the standard L 2 norm for square integrable functions. The gradient of the variational problem is defined as:
where ϕ s,t (x) denotes the position of the particle at time t while it is originally located at position x at time s, \( J_{t}^{0} = I_{0} \circ {\phi_{{t,0}}^v },J_{t}^{1} = I_{1} \circ \phi_{{t,1}}^v, \left| {D\phi_{{t,1}}^v} \right| \) and \( \nabla J_{t}^{0} \) are the determinant of Jacobian and the gradient of functions \( {D\phi_{{t,1}}^v} \) and \( J_{t}^{0} , \) respectively. Operator K is a compact self-adjoint operator such that for any smooth vector field a in V, K(L*L)a = a, for L* is the adjoint of L. Optimizing the velocity results in transformations that represent the shortest length path between each template and target particles. At the optimal geodesic flow, the variational gradient of Eq. (2) vanishes, which translates to:
In analogy with the laws of mechanics, (L*L)v t is called momentum that is proportional to α t (the magnitude of movement in the direction of gradient). At time t = 0 the initial momentum defines the initial trajectory that will transform template into the coordinate system of target image. Miller et al.19 have demonstrated, under the theory of geodesic shooting, that the scalar quantity α is conserved and can be computed at any other particular time greater than 0 given initial velocity and template anatomy are available. Geodesic evolution, starting from the optimum initial momentum, defines the entire path of evolution from template image to target.
Use of the LDDMM Algorithm to Compute Average Heart Shape
We have employed the LDDMM algorithm along with the theory of geodesic shooting to create average heart templates. The first step in this process is to estimate an optimal diffeomorphic transformation, using the LDDMM algorithm, which maps a provisional template to the individual target images in a cohort population. This step provides optimal initial velocity vector fields that will be used in the second step to compute average initial velocity vectors. The final step is to propagate these averaged initial velocity vectors along the direction of minimal energy path (geodesic). The final result is an evolved template that is in closer proximity to the true average shape. These steps are iterated until the magnitude of the averaged initial velocity approaches zero. In practice, due to noise interference in an inexact matching set, the magnitude of the averaged initial velocity reaches some minimum other than zero.
Statistical Analysis Using the LDDMM Derived Initial Momentum and PCA
The family of initial momentum vector fields \( Y = \left\{ {Lv_{0}^i } \right\}_{i = 1}^{N} \) (N = total number of subjects in ICM and NICM groups combined) on the C 2 manifold I 0 (template) belong to a set of zero-mean (mean subtracted) Gaussian random fields with an empirical covariance estimated as:
The initial momentum \( {Lv_{0}^i }\) can be extended using a complete orthonormal base \( \left\{ {\lambda_{k} ,\Upphi_{k} ,k = 1, \ldots ,N} \right\} \) as:
where \( \beta_{ik} = \sum\nolimits_{l} ({Lv_{0}^i (y_{l} ))^T\Upphi_{k} (y_{l} )dy_{l} } \) are the coefficients for the ith subject associated with kth basis function.12 Here dy l is the measure around voxel y l (i.e., voxel size). In this construction, λ k and Φ k are eigenvalues and the corresponding eigenvectors, respectively. The eigenvectors can be computed via singular value decomposition of the empirically estimated covariance.
Assuming the population under investigation consists of G groups, with each having N g subjects g = 1,…, G, then for each group g with all its subjects, let \( Z_{i}^{g} = \left[ {\beta_{i1}^{g} , \ldots ,\beta_{iN}^{g} } \right] \) be the vector coefficients associated with the eigenvectors as described before. Then the coefficient fields \( \left\{ {Z_{1}^{g} , \ldots ,Z_{{N_{g} }}^{g} } \right\} \) are samples of a random field with mean \( \bar{Z}^{g} . \) All groups have common, but unknown covariance Σ. If the subject population is grouped into N 1 ICM cases and N 2 NICM cases, then coefficient fields \( \left\{ {Z_{1}^{1} , \ldots ,Z_{{N_{1} }}^{1} } \right\} \) and \( \left\{ {Z_{1}^{2} , \ldots ,Z_{{N_{2} }}^{2} } \right\} \) are random processes with common covariance Σ and sample mean \( \bar{Z}^{1} \) and \( \bar{Z}^{2} \), respectively. The sample mean is computed as:
and the pooled sample covariance is:
Then the two groups of ventricular geometry are considered different in shape if the null hypothesis \( H_{o} :\bar{Z}^{1} = \bar{Z}^{2} \) is rejected with predetermined significance level (e.g., 0.05). To proceed, define the Hotelling T 2 statistics1 as:
Then, for all permutations of two given groups, new means and covariances are calculated employing Monte Carlo simulations to generate a large number of uniformly distributed random permutations. Collection of T 2 distributions for each permutation gives rise to an empirical distribution \( \hat{F} \) using:
to estimate F (M is the number of basis function that has been used to estimate principal coefficients). The null hypothesis is rejected when \( P = \int_{{T^{2} }}^{\infty } {\hat{F}(f)df} \) falls below a predetermined significant level.25
Rights and permissions
About this article
Cite this article
Ardekani, S., Weiss, R.G., Lardo, A.C. et al. Computational Method for Identifying and Quantifying Shape Features of Human Left Ventricular Remodeling. Ann Biomed Eng 37, 1043–1054 (2009). https://doi.org/10.1007/s10439-009-9677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9677-2