Abstract
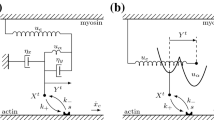
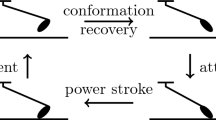
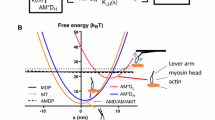
Molecular models of contractility in striated muscle require an integrated description of the action of myosin motors, firstly in the filament lattice of the half-sarcomere. Existing models do not adequately reflect the biochemistry of the myosin motor and its sarcomeric environment. The biochemical actin–myosin–ATP cycle is reviewed, and we propose a model cycle with two 4- to 5-nm working strokes, where phosphate is released slowly after the first stroke. A smaller third stroke is associated with ATP-induced detachment from actin. A comprehensive model is defined by applying such a cycle to all myosin-S1 heads in the half-sarcomere, subject to generic constraints as follows: (a) all strain-dependent kinetics required for actin–myosin interactions are derived from reaction-energy landscapes and applied to dimeric myosin, (b) actin–myosin interactions in the half-sarcomere are controlled by matching rules derived from the structure of the filaments, so that each dimer may be associated with a target zone of three actin sites, and (c) the myosin and actin filaments are treated as elastically extensible. Numerical predictions for such a model are presented in the following paper.







Similar content being viewed by others
References
Alberty R. A. (1968) Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphatase to adenosine diphosphate. J. Biol. Chem. 243:1337–1343
Bagshaw C. R. (1987) Are two heads better than one?. Nature 326:746–747
Bagshaw C. R., Trentham D. R. (1974) The characterization of myosin-product complexes and of product-release steps during the magnesium-ion dependent adenosine triphosphatase reaction. Biochem. J. 141:331–349
Berger C. E., Fagnant P. M., Heizmann S., Trybus K. M., Geeves M. A. (2001) ADP binding induces an asymmetry between the heads of unphosphorylated myosin. J. Biol. Chem. 276:23240–23245
Bordas J., Svennson A., Rothery M., Lowy J., Diakun G., Boesecke P. (1999) Extensibility and symmetry of actin filaments in contracting muscle. Biophys. J. 77:3197–3207
Brunello E., Reconditi M., Elangovan R., Linari M., Sun Y. -B., Narayanan T., Panine P., Piazzesi G., Irving M., Lombardi V. (2007) Skeletal muscle resists stretch by rapid binding of the second motor domain of myosin to actin. Proc. Natl. Acad. Sci. USA 104:20114–20119
Capitanio M., Canepari M., Cacciafesta P., Lombardi V., Cicchi R., Maffei M., Pavone F. S., Bottinelli R. (2006) Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proc. Natl. Acad. Sci. USA 103:87–92
Chase P. B., Macpherson J. M., Daniel T. L. (2004) A spatially explicit nanomechanical model of the half-sarcomere: myofilament compliance affects Ca2+-activation. Ann. Biomed. Eng. 32:1559–1568
Conibear P., Geeves M. A. (1998) Cooperativity between the two heads of rabbit skeletal muscle heavy meromyosin in binding to actin. Biophys. J. 75:926–937
Cooke R. (1997) Actomyosin interaction in striated muscle. Physiol. Rev. 77:597–671
Cooke R. (2004) The sliding filament model: 1972–2004. J. Gen. Physiol. 123:643–656
Cooke R., Franks K. (1980) All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry 19:2265–2269
Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. (1992) Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibers. J. Physiol. 451:247–278
Dominguez R., Freyzon Y., Trybus K. M., Cohen C. (1998) Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 94:559–571
Edman K. A. P. (1975) Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J. Physiol. (London) 246:255–275
Edman K. A. P., Mansson A., Caputo C. (1997) The biphasic force-velocity relationship in frog muscle fibres and its evaluation in terms of cross-bridge function. J. Physiol. 503 Pt 1:141–156
Eisenberg E., Hill T. L. (1985) Muscle contraction and free energy transduction in biological systems. Science 227:999–1006
Eisenberg E., Hill T. L., Chen Y. D. (1980) Cross-bridge model of muscle contraction. Biophys. J. 29:195–227
Ferenczi M. A., Bershitsky S. Y., Koubassova N., Siththanandan V., Helsby W. I., Panine P., Roessie M., Narayanan T., Tsaturyan A. K. (2005) The “Roll and Lock” mechanism of force generation in muscle. Structure 13:131–141
Finer J. T., Simmons R. M., Spudich J. A. (1994) Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368:113–119
Ford L. E., Huxley A. F., Simmons R. M. (1977) Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. 269:441–515
Ford L. E., Huxley A. F., Simmons R. M. (1981) The relation between stiffness and filament overlap in stimulated frog muscle fibres. J. Physiol. 311:218–249
Geeves M. A. (1991) The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem. J. 274:1–19
Geeves M. A., Fedorov R., Manstein D. J. (2005) Molecular mechanism of actomyosin-based motility. Cell. Mol. Life Sci. 62:1462–1477
Geeves M. A., Holmes K. C. (1999) Structural mechanism of muscle contraction. Annu. Rev. Biochem. 68:6876–728
Geeves M. A., Holmes K. C. (2005) The molecular mechanism of muscle contraction. Adv. Protein Chem. 71:161–169
Gollub J., Cremo C., Cooke R. (1996) ADP release produces a rotation of the neck region of smooth muscle myosin but not skeletal myosin. Nat. Struct. Biol. 3:796–802
Hanson J., Huxley H. E. (1953) Structural basis of the cross-striations in muscle. Nature 172:530–532
Hill T. L. (1974) Theoretical formalism for the sliding filament model of contraction of striated muscle, Part I. Prog. Phys. Mol. Biol. 28:267–340
Houdusse A., Szent-Gyorgi A. G., Cohen C. (2000) Three conformational states of scallop myosin S1. Proc. Nat. Acad. Sci. USA 97:11238–11243
Hudson S. L., Harford J. J., Denny R. C., Squire J. M. (1997) Myosin head configuration in relaxed fish muscle: resting state myosin heads must swing axially by up to 150A or turn upside down to reach rigor. J. Mol. Biol. 273:440–455
Hussan J., de Tombe P. P., Rice J. J. (2006) A spatially detailed myofilament model as a basis for large-scale biological simulations. IBM J. Res. Dev. 50:583–600
Huxley A. F. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7:255–318
Huxley H. E. (1969) The mechanism of muscular contraction. Science 164:1356–1366
Huxley A. F., Simmons R. M. (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Huxley H. E., Stewart A., Sosa H., Irving M. (1994) X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys. J. 67:2411–2421
Huxley A. F., Tideswell S. (1996) Filament compliance and tension transients in muscle. J. Muscle Res. Cell Motil. 17:507–511
Ito K., Liu X., Katayama E., Uyeda T. Q. P. (1999) Cooperativity between two heads of dictyostelium myosin II in in-vitro motility and ATP hydrolysis. Biophys. J. 76:985–992
Kabsch, W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. (1990) Atomic structure of the actin: DNase I complex. Nature 347:37–44
Knight P. J. (1996) Dynamic behavior of the head-tail junction of myosin. J. Mol. Biol. 255:269–274
Kohler J., Winkler G., Schulte I., Scholz T., McKenna W., Brenner B., Kraft T. (2002) Mutation of the myosin converter domain alters cross-bridge elasticity. Proc. Natl. Acad. Sci. USA 99:3557–3562
Kojima H., Ishijima A., Yanagida T. (1994) Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro manipulation. Proc. Natl. Acad. Sci. USA 91:12962–12966
Lauzon A. M., Fagnant P. M., Warshaw D. M., Trybus K. M. (2001) Coiled-coil unwinding at the smooth muscle myosin head-rod junction is required for optimal mechanical performance. Biophys. J. 80:1900–1904
Lewalle A., Steffen W., Stevenson O., Ouyang Z., Sleep J. (2008) Single molecule measurement of the stiffness of the rigor myosin head. Biophys. J. 94:2160–2169
Linari M., Caremani M., Piperio C., Brandt P., Lombardi V. (2007) Stiffness and fraction of myosin motors responsible for active force in permeabilised muscle fibers from rabbit psoas. Biophys. J. 92:2476–2490
Lombardi V., Piazzesi G., Reconditi M., Linari M., Lucii L., Stewart A., Sun Y. -B., Boesecke P., Naryanan T., Irving T., Irving M. (2004) X-ray diffraction studies of the contractile mechanism in single muscle fibres. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359:1883–1893
Luther P. K. (2004) Evolution of the muscle lattice in the vertebrate kingdom. Microsc. Anal. 18:9–11
Luther P. K., Squire J. M. (1990) Three-dimensional structure of the vertebrate muscle A-band. II The myosin filament superlattice. J. Mol. Biol. 141:409–439
Lymn R. W., Taylor E. W. (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10:4617–4624
Malnasi-Czizmadia A., Pearson D. S., Kovacs M., Woolley R. J., Geeves M. A., Bagshaw C. R. (2001) Kinetic resolution of a conformational transition and the ATP hydrolysis step using relaxation methods with a Dictyostelium myosin II mutant containing a single tryptophan residue. Biochemistry 40:12727–12737
Martyn D. A., Chase P. B., Regnier M., Gordon A. M. (2002) A simple model with myofilament compliance predicts activation-dependent crossbridge kinetics in skinned skeletal fibers. Biophys. J. 83:3425–3434
Matsubara I., Goldman Y. E., Simmons R. M. (1984) Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J. Mol. Biol. 173:15–33
Mijailovich S. M., Fredberg J. J., Butler J. P. (1996) On the theory of muscle contraction: filament extensibility and the development of isometric force and stiffness. Biophys. J. 71:1475–84
Millar N. C., Homsher E. (1990) The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. J. Biol. Chem. 265:20234–20240
Nishizaka T., Seo R., Tadakuma H., Kinosita K., Ishiwata S. (2000) Characterization of single actomyosin rigor bonds: load dependence of lifetime and mechanical properties. Biophys. J. 79:962–974
Nyitrai M., Rossi R., Adamek N., Pellegrino M. A., Bottinelli R., Geeves M. A. (2006) What limits the velocity of fast skeletal muscle contraction in mammals?. J. Mol. Biol. 355:432–442
Offer G., Knight P. J., Burgess S. A., Alamo L., Padron R. (2000) A new model for the surface arrangement of myosin molecules in tarantula thick filaments. J. Mol. Biol. 298:239–260
Pate E., Cooke R. (1989) A model of crossbridge action: the effects of ATP, ADP and Pi. J. Muscle Res. Cell Motil. 10:181–196
Pate E., Naber N., Matuska M., Franks-Skiba K., Cooke R. (1997) Opening of the myosin nucleotide triphosphate binding domain during the ATPase cycle. Biochem. 36:12155–12166
Piazzesi G., Lombardi V. (1995) A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 68:1966–1979
Piazzesi G., Lucii L., Lombardi V. (2002) The size and speed of the working stroke of muscle myosin and its dependence on the force. J. Physiol. 545 (Pt 1):145–151
Piazzesi G., Reconditi M., Linari M., Lucii L., Bianco P., Brunello E., Decostre V., Stewart A., Gore D. B., Irving T. C., Irving M., Lombardi V. (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131:784–795
Piazzesi G., Reconditi M., Linari M., Lucii L., Sun Y. -B., Narayanan T., Boesecke P., Lombardi V., Irving M. (2002) Mechanism of force generation by myosin heads in skeletal muscle. Nature 415:659–662
Ranatunga K. W., Coupland M. E., Mutungi G. (2002) An asymmetry in the phosphate dependence of tension transients induced by length perturbations in mammalian (rabbit psoas) muscle fibres. J. Physiol. 542(Pt 3):899–910
Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261:58–65
Rayment I., Rypniewski W. R., Schmidt-Base K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261:50–58
Regnier M., Morris C., Homsher E. (1995) Regulation of the cross-bridge transition from a weakly to a strongly bound state in skinned rabbit muscle fibers. Am. J. Physiol. 269:C1532–C1539
Sleep J. A., Hutton R. L. (1980) Exchange between inorganic phosphates and adenosine 5’-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19:1276–1283
Smith D. A., Sleep J. (2004) Mechanokinetics of rapid tension recovery in muscle: the myosin working stroke is followed by a slower release of phosphate. Biophys. J. 87:442–456
Smith D., Sleep J. (2006) Strain-dependent kinetics of the myosin working stroke, and how they could be probed with optical trap experiments. Biophys. J. 91:3359–3369
Steffen W., Smith D. A., Simmons R. M., Sleep J. (2001) Mapping the actin filament with myosin. Proc. Natl. Acad. Sci. USA 98:14949–14954
Steffen W., Smith D., Sleep J. (2003) The working stroke upon myosin-nucleotide complexes binding to actin. Proc. Natl. Acad. Sci. USA 100:6434–6439
Stein L. A., Schwarz R. P., Chock P. B., Eisenberg E. (1979) Mechanism of actomyosin triphosphatase. Evidence that adenosine 5’-triphosphatase hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry 18:3895–3909
Tanner B. C. W., Daniel T. L., Regnier M. (2007) Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Comp. Biol. 3:1195–1211
Taylor E. W. (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction. Crit. Rev. Biochem. 6:103–164
Taylor K. A., Schmitz H., Reedy M. C., Goldman Y. E., Franzini-Armstrong C., Sasaki H., Tregear R. T., Poole K., Lucaveche C., Edwards R. J., Chen L. F., Winkler H., Reedy M. K. (1999) Tomographic 3D reconstruction of quick-frozen, Ca2+ activated contracting insect flight muscle. Cell 99:421–431
Telley I. A., Denoth J. (2007) Sarcomere dynamics during muscular contraction and their implications to muscle function. J. Muscle Res. Cell Motil. 28:89–104
Tregear R. T., Reedy M. C., Goldman Y. E., Taylor K. A., Winkler H., Franzini-Armstrong C., Sasaki H., Lucaveche C., Reedy M. K. (2004) Cross-bridge number, position, and angle in target zones of cryofixed isometrically active insect flight muscle. Biophys. J. 86:3009–3019
Vandenboom R., Hannon J. D., Sieck G. C. (2002) Isotonic force modulates force redevelopment rate of intact frog muscle fibres: evidence for cross-bridge induced thin filament activation. J. Physiol. 543(Pt 2):555–566
Veigel C., Coluccio L. M., Jontes J. D., Sparrow J. C., Milligan R. A., Molloy J. E. (1999) The motor protein myosin-I produces its working stroke in two steps. Nature 398:530–533
Veigel C., Wang F., Sellers J. R., Molloy J. E. (2002) The gated gait of the processive molecular motor myosin V. Nat. Cell Biol. 4:59–65
Wakabayshi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys. J. 67:2422–2435
White H. D., Belknap B., Webb M. R. (1997) Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry 36:11828–11836
Whittaker, M., E. Wilson-Kubatek, J. E. Smith, L. Faust, R. A. Milligan, and H. L. Sweeney. A 35-A movement of smooth muscle myosin on ADP release. Nature 378:748–751, 1995
Wood J. E., Mann R. W. (1981) A sliding-filament cross-bridge ensemble model of muscle contraction for mechanical transients. Math. Biosciences 57:211–263
Acknowledgments
We acknowledge helpful discussions with many people, in particular P. Bennett, K. Burton, M. Irving, P. Luther, A. Mansson, H. Matheiss, G. Offer, V. Ovchinnikov, K.W. Ranatunga, and R.M. Simmons, who have contributed to the evolution of this paper. This work was carried out under a Bioengineering Research Partnership of the National Institutes of Health (Grant no. R01 AR048776). All authors acknowledge financial support from this program. M.A. Geeves is also supported by the Wellcome Trust (Program Grant 07002), and J. Sleep by the Medical Research Council (UK).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The Simplest Matching Conditions
For a given pair of nearest-neighbor myosin and actin filaments, the following prescription for matching heads to actin sites uses selection rules, defined by discrete cut-offs for the extent of longitudinal and azimuthal mismatches. The chosen site is assumed to be the center of a 3-site target zone. These rules are formulated for single myosin heads; they also apply to dimers with the obvious restriction that the two heads compete for a given site.
Longitudinal matching is achieved if \({\vert}x\vert < d_{\rm co}\), a cut-off distance, or
in the relaxed lattice. Heads in adjacent layers cannot compete for the same site if d co < a/6 (7.15 nm), a condition which seems eminently reasonable for myosin II.
Angular mismatches in the azimuthal plane involve the orientations of head and actin site relative to the rungs of the ladder (Fig. 7). The maximum head-ladder mismatch Δϕco should be above 20° to avoid a high fraction of inactive heads, and less than 30° to stop the head from exploring different F-actins, in which case the mapping would not be unique. The chosen actin site can be matched to the ladder with an angular tolerance of 13°, since adjacent monomeric sites on the same strand differ in angle by 2π/13 (27.7°). Ladders and F-actins in the unit cell are labeled by k and α as described in the main text. Let θkα be the angle of the kth ladder radiating from the F-actin labeled by α, so θk1 = (k−1)2π/3 and θk2 = θk1 + π (measured clockwise from the vertical in Fig. 7). In terms of the polar coordinates of Eqs. (9) and (10), these matching conditions are
provided angular differences are adjusted to [−π,π). Equation (A2a) gives the head-ladder map μ = μ k α(l) of the main text, and is tabulated in Table 1. This mapping is hard-wired by the lattice and the starting orientation of the crowns. Taken together, Eqs. (A1) and (A2) give the mapping n = n map(m,k,α) from head m to site n via the ladder (k,α); this mapping changes with the length of the sarcomere.
To summarize: as long as d co < 7 nm and Δϕco < 30°, the mapping is 1–0 or 1–1; not all heads can be mapped to sites, but two heads cannot be mapped to the same site. In detail: (i) Each head can be matched to only one ladder, so it cannot map to sites on different F-actins. (ii) Each ‘rung’ of the ladder can map to only actin site at most. (iii) Different heads on the same crown cannot select the same actin site because they select different ladders. (iv) Heads in adjacent layers on the same F-myosin cannot select the same site, although their 40° difference in azimuthal orientation may allow them to select the same ladder. (v) Heads on the three F-myosins surrounding a given F-actin generally address different actin sites, because angular matching to ladders whose orientations differ by 120° implies a mean spacing of 120/27.7 = 4.33 sites. This is sufficient to accommodate nonoverlapping zones of three sites. (vi) Because the 43-nm F-myosin repeat exceeds the 36-nm F-actin repeat, some heads cannot be longitudinally matched to any site (Fig. 1c).
The above matching rules are not unique. They were constructed to give tight angular matching of heads to sites via the azimuthal orientation of the ladder. With this condition, the range of longitudinal matching within the 7.0-nm limit can be determined kinetically by the strain dependence of the binding rate. Loose longitudinal binding occurs with the ‘swing-roll-lock’ mechanism when it \(\eta \gg 1\).
In the presence of filament motion, Eq. (A1) is replaced by
with x m,nα(t) defined by Eq. (15). In modeling, this condition should be applied to all detached heads after each time-integration step.
Rights and permissions
About this article
Cite this article
Smith, D., Geeves, M., Sleep, J. et al. Towards a Unified Theory of Muscle Contraction. I: Foundations. Ann Biomed Eng 36, 1624–1640 (2008). https://doi.org/10.1007/s10439-008-9536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9536-6