Abstract
The effect of spontaneous beat-to-beat mean arterial blood pressure (ABP) fluctuations and breath-to-breath end-tidal carbon dioxide \((P_{ETCO_2})\) and end-tidal oxygen \((P_{ETO_2})\) fluctuations on beat-to-beat cerebral blood flow velocity (CBFV) variations is studied using a multiple coherence function. Multiple coherence is a measure of the extent to which the output, CBFV, can be represented as a linear time invariant system of multiple input signals. Analysis of experimental measurements from 13 different healthy subjects reveal that, with additional inputs, \(P_{ETCO_2}\) and \(P_{ETO_2},\) the multiple coherence for frequencies < 0.05 Hz is significantly higher than the corresponding values obtained for univariate coherence with a single input of ABP. The result illustrates that the low value of univariate coherence at small frequencies may be due to the effects of \(P_{ETCO_2}\) and \(P_{ETO_2}\) fluctuations on CBFV variability. Moreover, it is also found that the transfer function between ABP and CBFV time series identified from previous univariate techniques at low frequencies can be modified by CO 2 and O 2 reactivity and no longer represents pressure autoregulation only. Multivariate system identification provides a technique of incorporating additional variability and recovering from this artifact. Finally, a physiologically based model and its linear transfer function are used as a simulation tool to investigate possible causes of low univariate coherence.








Similar content being viewed by others
Notes
Note that to remove baseline drift the signals were high-pass filtered as 0.005 Hz (see Methods). This explains the sharp drop in coherence at very low frequencies.
The model definitions can be found in Dutton et al.2.
In the following equations, the capital letter represents the parameter value, the small letter represents the nondimensional value respectively, which is the parameter value divided by its baseline value, denoted by the overbar.
References
Bendat J. S., Piersol A. G. (2000) Random Data: Analysis and Measurement Procedures. John Wiley & Sons Inc., New York, NY
Dutton K., Thompson S., Barraclough B. (1997) The Art of Control Engineering. Addison-Wesley Longman Publishing Co Inc., Boston, MA
Giller C. A., Mueller M. (2003) Linearity and non-linearity in cerebral hemodynamics. Med. Eng. Phys. 25(8):633–646
Goulden, C. H. Methods of Statistical Analysis (Wiley publications in statistics). Wiley, 1959.
Latka, M., M. Turalska, M. Glaubic-Latka, W. Kolodziej, D. Latka, and B. J. J. West. Phase dynamics in cerebral autoregulation. Am. J. Physiol. Heart Circ. Physiol., July 2005.
Liu Y., Birch A. A., Allen R. (2003) Dynamic cerebral autoregulation assessment using an arx model: comparative study using step response and phase shift analysis. Med. Eng. Phys. 25(8):647–653
Mitsis G. D., Ainslie P. N., Poulin M. J., Robbins P. A., Marmarelis V. Z. (2004) Nonlinear modeling of the dynamic effects of arterial pressure and blood gas variations on cerebral blood flow in healthy humans. Adv. Exp. Med. Biol. 551:259–265
Mitsis G. D., Poulin M. J., Robbins P. A., Marmarelis V. Z. (2004) Nonlinear modeling of the dynamic effects of arterial pressure and co2 variations on cerebral blood flow in healthy humans. IEEE Trans. Biomed. Eng. 51(11):1932–1943
Mitsis G. D., Zhang R., Levine B. D., Marmarelis V. Z. (2002) Modeling of nonlinear physiological systems with fast and slow dynamics. ii. Application to cerebral autoregulation. Ann. Biomed. Eng. 30(4):555–565
Nilsson, H., and C. Aalkjaer. Vasomotion: mechanisms and physiological importance. Mol. Interv., 3(2), March 2003.
Panerai R. B., Dawson S. L., Eames P. J., Potter J. F. (2001) Cerebral blood flow velocity response to induced and spontaneous sudden changes in arterial blood pressure. Am J Physiol Heart Circ Physiol 280(5):2162–2174
Panerai R. B., Dawson S. L., Potter J. F. (1999) Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am. J. Physiol. 277(3 Pt 2):1089–1099
Panerai R. B., Deverson S. T., Mahony P., Hayes P., Evans D. H. (1999) Effects of co2 on dynamic cerebral autoregulation measurement. Physiol Meas 20(3):265–275
Panerai, R. B., P. J. Eames, and J. F. Potter (2006) Multiple coherence of cerebral blood flow velocity in humans. Am. J. Physiol. Heart Circ. Physiol., 291(1), July 2006.
Panerai R. B., Simpson D. M., Deverson S. T., Mahony P., Hayes P., Evans D. H. (2000) Multivariate dynamic analysis of cerebral blood flow regulation in humans. IEEE Trans Biomed Eng 47(3):419–423
Paulson O. B., Strandgaard S., Edvinsson L. (1990) Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2(2):161–192
Payne S. J. (2006) A model of the interaction between autoregulation and neural activation in the brain. Mathematical Biosciences 204(2):260–281
Payne S. J., Tarassenko L. (2006) Combined transfer function analysis and modelling of cerebral autoregulation. Annals of Biomedical Engineering 34(5):847–858
Perreault E. J., Kirsch R. F., Acosta A. M. (1999) Multiple-input, multiple-output system identification for characterization of limb stiffness dynamics. Biol Cybern 80(5):327–337
Poulin M. J., Liang P. J., Robbins P. A. (1996) Dynamics of the cerebral blood flow response to step changes in end-tidal pco2 and po2 in humans. J Appl Physiol 81(3):1084–1095
Poulin M. J., Liang P.-J., Robbins P. A. (1998) Fast and slow components of cerebral blood flow response to step decreases in end-tidal pco2 in humans. J Appl Physiol 85(2):388–397
Renkin, E. M., and C. C. Michel, Handbook of Physiology: Section 2: The Cardiovascular System Volume IV, Parts 1 & 2: Microcirculation (Handbook of Physiology Revised Edition). An American Physiological Society Book, December 1983.
Rowley A. B., Payne S. J., Tachtsidis I., Ebden M. J., Whiteley J. P., Gavaghan D. J., Tarassenko L., Smith M., Elwell C. E., Delpy D. T. (2007) Synchronization between arterial blood pressure and cerebral oxyhaemoglobin concentration investigated by wavelet cross-correlation. Physiological Measurement 28(2):161–173
Tiecks F. P., Lam A. M., Aaslid R., Newell D. W. (1995) Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26(6):1014–1019
Ursino M., Lodi C. A. (1998) Interaction among autoregulation, co2 reactivity, and intracranial pressure: a mathematical model. Am J Physiol 274(5 Pt 2):1715–1728
Ursino M., Ter Minassian A., Lodi C. A., Beydon L. (2000) Cerebral hemodynamics during arterial and co(2) pressure changes: in vivo prediction by a mathematical model. Am J Physiol Heart Circ Physiol 279(5):2439–2455
Welch P. D. (1967) The use of fast fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Transactions on Audio and Electroacoustics 15:70–73
Zhang R., Zuckerman J. H., Giller C. A., Levine B. D. (1998) Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 274(1 Pt 2):233–241
Zhang R., Zuckerman J. H., Iwasaki K., Wilson T. E., Crandall C. G., Levine B. D. (2002) Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106(14):1814–1820
Acknowledgments
TP was supported by UK Research Councils though a Dorothy Hodgkin Postgraduate Awards (EP/P500923/1), ABR was supported by an EPSRC Life Sciences Interface Doctoral Training Centre studentship (GR/S58119/01), PNA was supported by Alberta Heritage Foundation for Medical Research and Focus-on-Stroke (Heart and Stroke Foundation of Canada, the Canadian Stroke Network, Canadian Institutes of Health Research, and AstraZeneca Canada) postdoctoral fellowships. The authors would like to thank funding support for data collection from the Alberta Heritage Foundation for Medical Research (AHFMR), the Heart and Stroke Foundation of Alberta, Northwest Territorites, & Nunavut, the Canadian Institutes of Health Research (CIHR), and the Canada Foundation for Innovation (CFI; New Opportunities program). We would also like to thank Professor Peter Robbins of University Laboratory of Physiology, University of Oxford, Dr. Georgios Mitsis of Department of Biomedical Engineering, University of Southern California, Professor David Gavaghan and Dr. Jonathan Whiteley of the Computational Biology Research Group, Oxford University for many helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a United Kingdom Research Funding Dorothy Hodgkin Postgraduate Award Scheme to T. Peng.
Appendix
Appendix
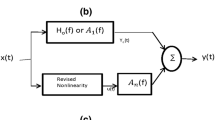
The schematic of the model used here is shown in Fig. 9. The full description of the system can be found in Payne17. All differential equations used in the model are summarized below:
Schematic of model. P a , systemic arterial pressure; R la , resistance of nonregulating arterial compartment; P 1, R sa , and C a , pressure, resistance, and compliance of regulating arterial compartment; R sv , resistance of capillary compartment and small veins; C v , venous compliance; P 2, venous pressure; P v and R lv , venous pressure and resistance of large veins, respectivly; P ic , intracranial pressure; C ic , intracranial compliance
In arterial compartment Footnote 3:
In capillary and venous compartments:
where k ven and P v1 are constants.
The feedback system:
where q is the microvascular CBF:
It is then assumed that the two feedback mechanisms act in a linearly additive manner:
and that x then modifies C a by means of the following sigmoidal relationship from:26
To derive the linear transfer function, small changes about the basal conditions are assumed using a Taylor series expansion. Since the resulting equations will all be linear, the Laplace transform is used to convert the differential equations into a transfer function. A detailed description of the linearization process can be found in Payne and Tarassenko.18 The differential equations governing the flow autoregulation above are thus linearized to the algebraic equations presented below:
in which A is the cross-sectional area of the MCA, which is assumed to be invariant here. Therefore, beat-to-beat changes in mean velocity may represent predominantly beat-to-beat changes in cerebral blood flow. Hence for small pressure changes, velocity changes will be:
After simplification and approximation, the transfer functions for V MCA is obtained as:
where the relevant nondimensional parameters and time constants are:
The differential equations governing CO 2 cerebrovascular reactivity are almost the same as those governing flow autoregulation. The difference exists only in two equations: Equation (19) becomes:
Since there is no arterial pressure changes, the compliance equation [Eq. (28)] becomes:
After simplification, the transfer function V MCA to CO 2 changes is obtained as:
where the definitions of [α1, α2, β1, τ q , τ a ] are the same as before.
Rights and permissions
About this article
Cite this article
Peng, T., Rowley, A.B., Ainslie, P.N. et al. Multivariate System Identification for Cerebral Autoregulation. Ann Biomed Eng 36, 308–320 (2008). https://doi.org/10.1007/s10439-007-9412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9412-9