Abstract
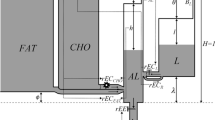
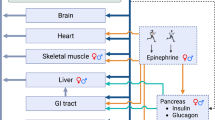
A mathematical model of the whole-body metabolism is developed to predict fuel homeostasis during exercise by using hormonal control over cellular metabolic processes. The whole body model is composed of seven tissue compartments: brain, heart, liver, GI (gastrointestinal) tract, skeletal muscle, adipose tissue, and “other tissues”. Each tissue compartment is described by dynamic mass balances and major cellular metabolic reactions. The glucagon–insulin controller is incorporated into the whole body model to predict hormonal changes during exercise. Moderate [150 W power output at 60% of peak oxygen consumption (VO2max)] exercise for 60 min was implemented by increasing ATP utilization rates in heart and skeletal muscle. Arterial epinephrine level was given as an input function, which directly affects heart and skeletal muscle metabolism and indirectly other tissues via glucagon–insulin controller. Model simulations were validated with experimental data from human exercise studies. The exercise induced changes in hormonal signals modulated metabolic flux rates of different tissues in a coordinated way to achieve glucose homeostasis, demonstrating the efficacy of hormonal control over cellular metabolic processes. From experimental measurements of whole body glucose balance and arterial substrate concentrations, this model could predict the dynamic changes of hepatic glycogenolysis and gluconeogenesis, which are not easy to measure experimentally, suggesting the higher contribution of glycogenolysis (∼75%). In addition, it could provide dynamic information on the relative contribution of carbohydrates and lipids for fuel oxidation in skeletal muscle. Model simulations indicate that external fuel supplies from other tissue/organ systems to skeletal muscle become important for prolonged exercise emphasizing the significance of interaction among tissues. In conclusion, this model can be used as a valuable complement to experimental studies due to its ability to predict what is difficult to measure directly, and usefulness to provide information about dynamic behaviors.









Similar content being viewed by others
References
Abumrad N. N., Cherrington A. D., Williams P. E., Lacy W. W., Rabin D. (1982). Absorption and disposition of a glucose load in the conscious dog. Am. J. Physiol. 242:E398–E406
Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. (1974). Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J. Clin. Invest. 53:1080–1090
Aubert A., Costalat R., Duffau H., Benali H. (2002). Modeling of pathophysiological coupling between brain electrical activation, energy metabolism and hemodynamics: insights for the interpretation of intracerebral tumor imaging. Acta Biotheor. 50:281–295
Auricchio A., Zhou R., Wilson J. M., Glickson J. D. (4–24–2001). In vivo detection of gene expression in liver by 31P nuclear magnetic resonance spectroscopy employing creatine kinase as a marker gene. Proc. Natl. Acad. Sci. USA 98:5205–5210
Barzilai N., Rossetti L. (1993). Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J. Biol. Chem. 268:25019–25025
Bergman B. C., Brooks G. A. (1999) Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 86:479–487
Bergman B. C., Butterfield G. E., Wolfel E. E., Casazza G. A., Lopaschuk G. D., Brooks G. A. (1999). Evaluation of exercise and training on muscle lipid metabolism. Am. J. Physiol. 276:E106–E117
Bergman B. C., Butterfield G. E., Wolfel E. E., Lopaschuk G. D., Casazza G. A., Horning M. A., Brooks G. A. (1999). Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 277:E81–E92
Bergman B. C., Horning M. A., Casazza G. A., Wolfel E. E., Butterfield G. E., Brooks G. A. (2000). Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol Endocrinol. Metab. 278:E244–E251
Bergman B. C., Wolfel E. E., Butterfield G. E., Lopaschuk G. D., Casazza G. A., Horning M. A., Brooks G. A. (1999). Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 87:1684–1696
Bergman R. N., Ider Y. Z., Bowden C. R., Cobelli C. (1979). Quantitative estimation of insulin sensitivity. Am. J. Physiol. 236:E667–E677
Bjorkman O., Eriksson L. S., Nyberg B., Wahren J. (1990). Gut exchange of glucose and lactate in basal state and after oral glucose ingestion in postoperative patients. Diabetes. 39:747–751
Bode J. C., Zelder O., Rumpelt H. J., Wittkamp U. (1973). Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur. J. Clin. Invest. 3:436–441
Brooks G. A., Mercier J. (1994). Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J. Appl. Physiol. 76:2253–2261
Brundin T., Branstrom R., Wahren J. (1996). Effects of oral vs. i.v. glucose administration on splanchnic and extrasplanchnic O2 uptake and blood flow. Am. J. Physiol. 271:E496–E504
Brundin T., Wahren J. (1993). Whole body and splanchnic oxygen consumption and blood flow after oral ingestion of fructose or glucose. Am. J. Physiol. 264:E504–E513
Cabrera M. E., Saidel G. M., Kalhan S. C. (1998). Role of O2 in regulation of lactate dynamics during hypoxia: mathematical model and analysis. Ann. Biomed. Eng. 26:1–27
Cabrera M. E., Saidel G. M., Kalhan S. C. (1999). Lactate metabolism during exercise: analysis by an integrative systems model. Am. J. Physiol. 277:R1522–R1536
Chen J., Gollnick P. D. (1994). Effect of exercise on hexokinase distribution and mitochondrial respiration in skeletal muscle. Pflugers Arch. 427:257–263
Cruz J. (2003). Expensive cerebral blood flow measurements alone are useless and misinformative in comatose patients: a comprehensive alternative. Arq Neuropsiquiatr. 61:309–312
Dash R. K., Bassingthwaighte J. B. (2006). Simultaneous blood-tissue exchange of oxygen, carbon dioxide, bicarbonate and hydrogen ion. Ann. Biomed. Eng. 34:1129–1148
Dash, R. K., Y. Li, G. M. Saidel, and M. E. Cabrera. Metabolic dynamics in skeletal muscle during acute reduction in blood flow and oxygen supply to mitochondria: In-silico studies. Am. J. Physiol Cell Physiol. 2006 (provisionally accepted).
Davis S. N., Galassetti P., Wasserman D. H., Tate D. (2000). Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J. Clin. Endocrinol. Metab. 85:224–230
Delzenne N. M., Kok N. N. (1999). Biochemical basis of oligofructose-induced hypolipidemia in animal models. J. Nutr. 129:1467S–1470S
Ekberg K., Landau B. R., Wajngot A., Chandramouli V., Efendic S., Brunengraber H., Wahren J. (1999). Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48:292–298
Ercan-Fang N., Gannon M. C., Rath V. L., Treadway J. L., Taylor M. R., Nuttall F. Q. (2002). Integrated effects of multiple modulators on human liver glycogen phosphorylase a. Am. J. Physiol Endocrinol. Metab. 283:E29–E37
Frayn K. N., Shadid S., Hamlani R., Humphreys S. M., Clark M. L., Fielding B. A., Boland O., Coppack S. W. (1994). Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am. J. Physiol. 266:E308–E317
Friedlander A. L., Casazza G. A., Horning M. A., Huie M. J., Brooks G. A. (1997). Training-induced alterations of glucose flux in men. J. Appl. Physiol. 82:1360–1369
Friedlander A. L., Casazza G. A., Horning M. A., Usaj A., Brooks G. A. (1999). Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J. Appl. Physiol. 86:2097–2105
Galbo H., Richter E. A., Hilsted J., Holst J. J., Christensen N. J., Henriksson J. (1977). Hormonal regulation during prolonged exercise. Ann. N. Y. Acad. Sci. 301:72–80
Gerich J. E. (2000). Physiology of glucose homeostasis. Diabetes Obes. Metab. 2:345–350
Gerich J.E. (1993). Control of glycaemia. Baillieres Clin. Endocrinol. Metab. 7:551–586
Gross R. C., Eigenbrodt E. H., Farquhar J. W. (1967). Endogenous triglyceride turnover in liver and plasma of the dog. J. Lipid Res. 8:114–125
Harvey W. D., Faloona G. R., Unger R. H. (1974). The effect of adrenergic blockade on exercise-induced hyperglucagonemia. Endocrinology 94:1254–1258
Henderson G. C., Horning M. A., Lehman S. L., Wolfel E. E., Bergman B. C., Brooks G. A. (2004). Pyruvate shuttling during rest and exercise before and after endurance training in men. J. Appl. Physiol. 97:317–325
Hirsch I. B., Marker J. C., Smith L. J., Spina R. J., Parvin C. A., Holloszy J. O., Cryer P. E. (1991). Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am. J. Physiol. 260:E695–E704
Hudetz A.G. (1999). Mathematical model of oxygen transport in the cerebral cortex. Brain Res. 817:75–83
Hultman E., Nilsson L. H., Sahlin K. (1975). Adenine nucleotide content of human liver. Normal values and fructose-induced depletion. Scand. J. Clin. Lab Invest. 35:245–251
Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., Shulman G. I. (2000). Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49:2063–2069
Ide K., Horn A., Secher N. H. (1999). Cerebral metabolic response to submaximal exercise. J. Appl. Physiol. 87:1604–1608
Ide, K., I. K. Schmalbruch, B. Quistorff, A. Horn, and N. H. Secher. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J. Physiol. 522 Pt 1:159–164, 2000
Jensen M. D. (1999). Regional glycerol and free fatty acid metabolism before and after meal ingestion. Am. J. Physiol. 276:E863–E869
Karpe F., Fielding B. A., Ardilouze J. L., Ilic V., Macdonald I. A., Frayn K. N. (2002). Effects of insulin on adipose tissue blood flow in man. J. Physiol. 540:1087–1093
Kemp B. E., Mitchelhill K. I., Stapleton D., Michell B. J., Chen Z. P., Witters L. A. (1999). Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 24:22–25
Klein S., Wolfe R. R. (1990). Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J. Clin. Invest. 86:1403–1408
Korzeniewski B., Liguzinski P. (2004). Theoretical studies on the regulation of anaerobic glycolysis and its influence on oxidative phosphorylation in skeletal muscle. Biophys. Chem. 110:147–169
Krebs H. A., Hems R., Weidemann M. J., Speake R. N. (1966). The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem. J. 101:242–249
Krudys K. M., Dodds M. G., Nissen S. M., Vicini P. (2005). Integrated model of hepatic and peripheral glucose regulation for estimation of endogenous glucose production during the hot IVGTT. Am. J. Physiol Endocrinol. Metab. 288:E1038–E1046
Lambeth M. J., Kushmerick M. J. (2002). A computational model for glycogenolysis in skeletal muscle. Ann. Biomed. Eng. 30:808–827
Landau B. R., Wahren J., Chandramouli V., Schumann W. C., Ekberg K., Kalhan S. C. (1995). Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J. Clin. Invest. 95:172–178
Lund S., Holman G. D., Schmitz O., Pedersen O. (1995). Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc. Natl. Acad. Sci. USA 92:5817–5821
Luyckx A. S., Lefebvre P. J. (1974). Mechanisms involved in the exercise-induced increase in glucagon secretion in rats. Diabetes 23:81–93
Martin W. H. III, Dalsky G. P., Hurley B. F., Matthews D. E., Bier D. M., Hagberg J. M., Rogers M. A., King D. S., Holloszy J. O. (1993). Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am. J. Physiol. 265:E708–E714
Mikines K. J., Richter E. A., Dela F., Galbo H. (1991). Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J. Appl. Physiol. 70:1245–1254
Miyoshi H., Shulman G. I., Peters E. J., Wolfe M. H., Elahi D., Wolfe R. R. (1988). Hormonal control of substrate cycling in humans. J. Clin. Invest. 81:1545–1555
Morikawa S., Inubushi T., Takahashi K., Ishii H., Ozawa K. (1998). Gluconeogenesis and phosphoenergetics in rat liver during endotoxemia. J. Surg. Res. 74:179–186
Musi N., Goodyear L.J. (2003). AMP-activated protein kinase and muscle glucose uptake. Acta Physiol Scand. 178:337–345
Nybo L., Nielsen B., Blomstrand E., Moller K., Secher N. (2003). Neurohumoral responses during prolonged exercise in humans. J. Appl. Physiol. 95:1125–1131
Odland L. M., Heigenhauser G. J., Wong D., Hollidge-Horvat M. G., Spriet L. L. (1998). Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Am. J. Physiol. 274:R894–R902
Petersen K. F., Krssak M., Navarro V., Chandramouli V., Hundal R., Schumann W. C., Landau B. R., Shulman G. I. (1999). Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am. J. Physiol. 276:E529–E535
Petersen K. F., Price T., Cline G. W., Rothman D. L., Shulman G. I. (1996). Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am. J. Physiol. 270:E186–E191
Putman C. T., Jones N. L., Hultman E., Hollidge-Horvat M. G., Bonen A., McConachie D. R., Heigenhauser G. J. (1998). Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am. J. Physiol. 275:E132–E139
Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C. M., Kalhan S. C., Tilghman S. M., Hanson R. W. (2003) Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 278:30413–30416
Richter E. A., Nielsen J. N., Jorgensen S. B., Frosig C., Wojtaszewski J. F. (2003). Signalling to glucose transport in skeletal muscle during exercise. Acta Physiol Scand. 178:329–335
Salem J. E., Saidel G. M., Stanley W. C., Cabrera M. E. (2002). Mechanistic model of myocardial energy metabolism under normal and ischemic conditions. Ann. Biomed. Eng. 30:202–216
Samols E., Weir G. C. (1979). Adrenergic modulation of pancreatic A, B, and D cells alpha-Adrenergic suppression and beta-adrenergic stimulation of somatostatin secretion, alpha-adrenergic stimulation of glucagon secretion in the perfused dog pancreas. J. Clin. Invest. 63:230–238
Saunders P. T., Koeslag J. H., Wessels J. A. (1998). Integral rein control in physiology. J. Theor. Biol. 194:163–173
Saunders P. T., Koeslag J. H., Wessels J. A. (2000). Integral rein control in physiology II: a general model. J. Theor. Biol. 206:211–220
Shulman G.I. (2004). Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda.). 19:183–190
Shulman G. I., Ladenson P. W., Wolfe M. H., Ridgway E. C., Wolfe R. R. (1985). Substrate cycling between gluconeogenesis and glycolysis in euthyroid, hypothyroid, and hyperthyroid man. J. Clin. Invest. 76:757–764
Sigal R. J., Kenny G. P., Wasserman D. H., Castaneda-Sceppa C. (2004). Physical activity/exercise and type 2 diabetes. Diabetes Care 27:2518–2539
Simoncikova P., Wein S., Gasperikova D., Ukropec J., Certik M., Klimes I., Sebokova E. (2002). Comparison of the extrapancreatic action of gamma-linolenic acid and n-3 PUFAs in the high fat diet-induced insulin resistance [corrected]. Endocr. Regul. 36:143–149
Sindelar D. K., Chu C. A., Venson P., Donahue E. P., Neal D. W., Cherrington A. D. (1998). Basal hepatic glucose production is regulated by the portal vein insulin concentration. Diabetes 47:523–529
Stanley W. C., Gertz E. W., Wisneski J. A., Neese R. A., Morris D. L., Brooks G. A. (1986). Lactate extraction during net lactate release in legs of humans during exercise. J. Appl. Physiol. 60:1116–1120
Strisower E. H., Kohler G. D., Chaikoff I. L. (1952). Incorporation of acetate carbon into glucose by liver slices from normal and alloxan-diabetic rats. J. Biol. Chem. 198:115–126
Tiessen R. G., Rhemrev-Boom M. M., Korf J. (2002). Glucose gradient differences in subcutaneous tissue of healthy volunteers assessed with ultraslow microdialysis and a nanolitre glucose sensor. Life Sci. 70:2457–2466
Trimmer J. K., Schwarz J. M., Casazza G. A., Horning M. A., Rodriguez N., Brooks G. A. (2002). Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J. Appl. Physiol. 93:233–241
Turcotte L. P., Richter E. A., Kiens B. (1992). Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am. J. Physiol. 262:E791–E799
Vicini P., Zachwieja J. J., Yarasheski K. E., Bier D. M., Caumo A., Cobelli C. (1999). Glucose production during an IVGTT by deconvolution: validation with the tracer-to-tracee clamp technique. Am. J. Physiol. 276:E285-E294
Wahren J., Felig P., Ahlborg G., Jorfeldt L. (1971). Glucose metabolism during leg exercise in man. J. Clin. Invest. 50:2715–2725
Wahren J., Hagenfeldt L., Felig P. (1975). Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J. Clin. Invest. 55:1303–1314
Warram J. H., Martin B. C., Krolewski A. S., Soeldner J. S., Kahn C. R. (1990). Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann. Intern. Med. 113:909–915
Wasserman D. H., Ayala J. E. (2005). Interaction of physiological mechanisms in control of muscle glucose uptake. Clin. Exp. Pharmacol. Physiol. 32:319–323
Wasserman D. H., Cherrington A. D. (1991) Hepatic fuel metabolism during muscular work: role and regulation. Am. J. Physiol. 260:E811–E824
Wasserman D. H., Halseth A. E. (1998). An overview of muscle glucose uptake during exercise. Sites of regulation. Adv. Exp. Med. Biol. 441:1–16
Wasserman D. H., Vranic M. (1986). Interaction between insulin and counterregulatory hormones in control of substrate utilization in health and diabetes during exercise. Diabetes Metab. Rev. 1:359–384
Wisneski J. A., Gertz E. W., Neese R. A., Gruenke L. D., Morris D. L., Craig J. C. (1985). Metabolic fate of extracted glucose in normal human myocardium. J. Clin. Invest. 76:1819–1827
Zhou L., Salem J. E., Saidel G. M., Stanley W. C., Cabrera M. E. (2005). Mechanistic model of cardiac energy metabolism predicts localization of glycolysis to cytosolic subdomain during ischemia. Am. J. Physiol Heart Circ. Physiol. 288:H2400–H2411
Zhou L., Stanley W. C., Saidel G. M., Yu X., Cabrera M. E. (2005). Regulation of lactate production at the onset of ischemia is independent of mitochondrial NADH/NAD+: insights from in silico studies. J. Physiol. 569:925–937
Acknowledgments
This research was supported by NIH grant (P50-GM-66309) from the National Institute of General Medical Sciences for developing the Center for Modeling Integrated Metabolic Systems. The authors appreciate the helpful suggestions from John Kirwan and Ranjan Dash, and the effort to construct the physiological database for this whole body model by Jennifer Salem, Valerie Jurkovich, and Haiying Zhou.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix
Appendix 1. Kinetic equations for the metabolic reactions in tissue x.
Appendix 2. Dynamic mass balance equations in tissue x.
Rights and permissions
About this article
Cite this article
Kim, J., Saidel, G.M. & Cabrera, M.E. Multi-Scale Computational Model of Fuel Homeostasis During Exercise: Effect of Hormonal Control. Ann Biomed Eng 35, 69–90 (2007). https://doi.org/10.1007/s10439-006-9201-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9201-x