Abstract
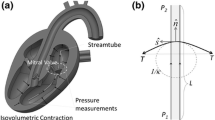
Characterization of the mechanical properties of the native mitral valve leaflets at physiological strain rates is a critical step in improving our understanding of MV function and providing experimental data for dynamic constitutive models. We explored, for the first time, the effects of strain rate (from quasi-static to physiologic) on the biaxial mechanical properties of the native mitral valve anterior leaflet (MVAL). A novel high-speed biaxial testing device was developed, capable of achieving in vitro strain rates reported for the MVAL (Sacks et al., Ann. Biomed. Eng. 30(10):1280–1290, 2002). Porcine MVAL specimens were loaded to physiological load levels with cycle periods of 15, 1, 0.5, 0.1, and 0.05 s. The resulting loading stress–strain responses were found to be remarkably independent of strain rate. The hysteresis, defined as the fraction of the membrane strain energy between the loading and unloading curves tension-areal stretch curves, was low (∼12%) and did not vary with strain rate. The results of the present work indicated that MVAL tissues exhibit complete strain rate insensitivity at and below physiological strain rates under physiological loading conditions. These novel results suggest that experimental tests utilizing quasi-static strain rates are appropriate for constitutive model development for mitral valve tissues. The mechanisms underlying this quasi-elastic behavior are as yet unknown, but are likely an important functional aspect of native mitral valve tissues and clearly warrant further study.









Similar content being viewed by others
REFERENCES
Alfieri, O., and F. Maisano. An effective technique to correct anterior mitral leaflet prolapse. J. Card. Surg. 14(6):468–470, 1999.
Billiar, K. L., and M. S. Sacks. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp–Part I: Experimental results. J. Biomech. Eng. 122(1):23–30, 2000.
Borer, J. S., and K. Kupfer. Mitral regurgitation: Current treatment options and their selection. Curr. Treat. Options Cardiovasc. Med. 6(6):509–517, 2004.
Carew, E. O., A. Garg, J. E. Barber, and I. Vesely. Stress relaxation preconditioning of porcine aortic valves. Ann. Biomed. Eng. 32(4):563–572, 2004.
Cole, W. G., D. Chan, A. J. Hickey, and D. E. Wilcken. Collagen composition of normal and myxomatous human mitral heart valves. Biochem. J. 219(2):451–460, 1984.
Curtis, M. B., and D. V. Priola. Mechanical properties of the canine mitral valve: Effects of autonomic stimulation. Am. J. Physiol. 262(1 Pt 2): H56–H62, 1992.
David, T. E., M. Komeda, C. Pollick, and R. J. Burns. Mitral valve annuloplasty: The effect of the type on left ventricular function. Ann. Thorac Surg. 47(4):524–527, 1989; discussion 527–528.
Doehring, T. C., E. O. Carew, and I. Vesely. The effect of strain rate on the viscoelastic response of aortic valve tissue: A direct-fit approach. Ann. Biomed. Eng. 32(2):223–2302, 2004.
El Khoury, G., P. Noirhomme, R. Verhelst, J. Rubay, and R. Dion. Surgical repair of the prolapsing anterior leaflet in degenerative mitral valve disease. J. Heart Valve Dis. 9(1):75–80, 2000; discussion 81.
Gilbert, T. W., M. S. Sacks, J. S. Grashow, S. L. Y. Woo, M. B. Chancellor, and S. F. Badylak. Fiber kinematics of small intestinal submucosa under uniaxial and biaxial stretch. J. Biomech. Eng., in press.
Hashim, S. R., A. Fontaine, S. He, R. A. Levine, and A. P. Yoganathan. A three-component force vector cell for in vitro quantification of the force exerted by the papillary muscle on the left ventricular wall. J. Biomech. 30(10):1071–1075, 1997.
Haut, R. C. Age-dependent influence of strain rate on the tensile failure of rat-tail tendon. J. Biomech. Eng. 105(3):296–299, 1983.
He, S., A. A. Fontaine, E. Schwammenthal, A. P. Yoganathan, and R. A. Levine. Integrated mechanism for functional mitral regurgitation: Leaflet restriction versus coapting force: In vitro studies. Circulation 96(6):1826–1834, 1997.
He, Z., M. S. Sacks, L. Baijens, S. Wanant, P. Shah, and A. P. Yoganathan. Effects of papillary muscle position on in vitro dynamic strain on the porcine mitral valve. J. Heart Valve Dis. 12(4):488–494, 2003.
Kreindel, M. S., W. A. Schiavone, H. M. Lever, and D. Cosgrove. Systolic anterior motion of the mitral valve after carpentier ring valvuloplasty for mitral valve prolapse. Am. J. Cardiol. 57(6):408–412, 1986.
Kunzelman, K. S., M. S. Sacks, R. P. Cochran, and R. C. Eberhart. Mitral valve leaflet collagen distribution by laser analysis. In: Proceedings of the Seventh Southern Biomedical Engineering Conference, TX: Dallas, 1988, pp. 82–85.
Lam, J. H., N. Ranganathan, E. D. Wigle, and M. D. Silver. Morphology of the human mitral valve. I. Chordae tendineae: A new classification. Circulation 41(3):449–458, 1970.
Lanir, Y. A structural theory for the homogeneous biaxial stress–strain relationships in flat collageneous tissues. J. Biomech. 12:423–436, 1979.
Lanir, Y. Constitutive equations for fibrous connective tissues. J. Biomech. 16:1–12, 1983.
Lee, J. M., D. W. Courtman, and D. R. Boughner. The glutaraldehyde-stabilized porcine aortic valve xenograft. I. Tensile viscoelastic properties of the fresh leaflet material. J. Biomed. Mater. Res. 18:61–77, 1984.
Leeson-Dietrich, J., D. Boughner, and I. Vesely. Porcine pulmonary and aortic valves: A comparison of their tensile viscoelastic properties at physiological strain rates. J. Heart Valve Dis. 4:88–94, 1995.
Liao, J., L. Yang, J. Grashow, and M. S. Sacks. Molecular orientation of collagen in intact planar connective tissues under biaxial stretch. Acta Biomater. 1(1), 2004.
Lim, K. O., and D. R. Boughner. Low frequency dynamic viscoelastic properties of human mitral valve tissue. Cardiovasc. Res. 10(4):45–54, 1976.
Lis, Y., M. C. Burleigh, D. J. Parker, A. H. Child, J. Hogg, and M. J. Davies. Biochemical characterization of individual normal, floppy and rheumatic human mitral valves. Biochem. J. 244(3):597–603, 1987.
Lydon, C., J. Crisco, M. Panjabi, and M. Galloway. Effect of elongation rate on the failure properties of the rabbit anterior cruciate ligament. Clin. Biomech. (Bristol, Avon) 10(8):428–433, 1995.
May-Newman, K., and F. C. Yin. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am. J. Physiol. 269(4 Pt 2):H1319–H1327, 1995.
May-Newman, K., and F. C. Yin. A constitutive law for mitral valve tissue. J. Biomech. Eng. 120(1):38–47, 1998.
Merryman, W. D., H. Y. S. Huang, F. J. Schoen, and M. S. Sacks. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J. Biomech., 39(1):88–96, 2006.
Naimark, W. A. Structure/function relations in mammalian pericardial tissue: Implications for comparative and developmental physiology, University of Toronto, 1995.
Naimark, W. A., J. M. Lee, H. Limeback, and D. Cheung. Correlation of structure and viscoelastic properties in the pericardia of four mammalian species. Am. J. Physiol. 263(32):H1095–H1106, 1992.
Naimark, W. A., S. D. Waldman, R. J. Anderson, B. Suzuki, C. A. Pereira, and J. M. Lee. Thermomechanical analysis of collagen crosslinking in the developing lamb pericardium. Biorheology 35(1):1–16, 1998.
Ormiston, J. A., P. M. Shah, C. Tei, and M. Wong. Size and motion of the mitral valve annulus in man. I. A two-dimensional echocardiographic method and findings in normal subjects. Circulation 64(1):113–120, 1981.
Otto, C. M. Valvular Heart Disease. Philadelphia: Saunders, 2004.
Perier, P., B. Clausnizer, and K. Mistarz. Carpentier “sliding leaflet” technique for repair of the mitral valve: Early results. Ann. Thoracic Surg. 57:383–386, 1994.
Perloff, J. K., and W. C. Roberts. The mitral apparatus: Functional anatomy of mitral regurgitation. Circulation 46:227–239, 1972.
Ranganathan, N., J. H. Lam, E. D. Wigle, and M. D. Silver. Morphology of the human mitral valve. II. The value leaflets. Circulation 41(3):459–467, 1970.
Sacks, M. S. Biaxial mechanical evaluation of planar biological materials. J. Elasticity 61:199–246, 2000.
Sacks, M. S., Z. He, L. Baijens, S. Wanant, P. Shah, H. Sugimoto, and A. P. Yoganathan. Surface strains in the anterior leaflet of the functioning mitral valve. Ann. Biomed. Eng. 30(10):1281–1290, 2002.
Silverman, M. E., and J. W. Hurst. The mitral complex. Interaction of the anatomy, physiology, and pathology of the mitral annulus, mitral valve leaflets, chordae tendineae, and papillary muscles. Am. Heart J. 76(3):399–418, 1968.
Smedira, N. G., R. Selman, D. M. Cosgrove, P. M. McCarthy, B. W. Lytle, P. C. Taylor, C. Apperson-Hansen, R. W. Stewart, and F. D. Loop. Repair of anterior leaflet prolapse: Chordal transfer is superior to chordal shortening. J. Thorac Cardiovasc. Surg. 112(2):287–291, 1996; discussion 291–292.
Woo, S. L., M. A. Gomez, and W. H. Akeson. The time and history-dependent viscoelastic properties of the canine medical collateral ligament. J. Biomech. Eng. 103(4):293–298, 1981.
Woo, S. L. Y., C. A. Orlando, J. F. Camp, and W. H. Akeson. Effects of postmortem storage by freezing on ligament tensile behavior. J. Biomech. 19:399–404, 1994.
Yacoub, M. H., and L. H. Cohn. Novel approaches to cardiac valve repair: From structure to function: Part II. Circulation 109(9):1064–1072, 2004.
ACKNOWLEDGMENTS
This work was funded by NIH grant HL-52009. MSS is an Established Investigator of the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grashow, J.S., Yoganathan, A.P. & Sacks, M.S. Biaixal Stress–Stretch Behavior of the Mitral Valve Anterior Leaflet at Physiologic Strain Rates. Ann Biomed Eng 34, 315–325 (2006). https://doi.org/10.1007/s10439-005-9027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-9027-y