Abstract
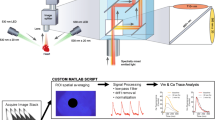
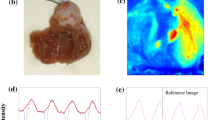
Cardiac action potentials have been measured with single-photon excitation (SPE) of transmembrane voltage-sensitive fluorescent dye. Two-photon excitation (TPE) may have advantages for localization and depth of the tissue region from which the action potential is measured. However measurements of action potentials with SPE have not been demonstrated. We sought to develop a method for TPE of di-4-ANEPPS and test whether the method yields voltage-dependent fluorescence in cardiac tissue. We modified our SPE and ratiometric fluorescence recording system to use a femtosecond pulsed near-infrared laser. Modifications were made to enhance fluorescence collection efficiency and to block infrared laser light from entering the fluorescence collection system. Fluorescence was collected simultaneously in green (510–570nm) and red (590–700nm) wavelength bands. Action potentials were observed in the ratio of the green signal to the red signal, but were not observed above the noise level in either of the individual signals. Incorporation of a common-mode noise subtraction method revealed action potentials in green and red signals. We also found that the di-4-ANEPPS fluorescence emission spectrum for TPE at 930nm was similar to the emission spectrum for SPE at 488nm. The multiphoton method may be beneficial for highly localized cardiac optical measurements.
Similar content being viewed by others
References
Baxter, W. T., S. F. Mironov, A. V. Zaitsev, J. Jalife, and A. M. Pertsov. Visualizing excitation waves inside cardiac muscle using transillumination. Biophys. J. 80:516–530, 2001.
Bestvater, F., E. Spiess, G. Stobrawa, M. Hacker, T. Feurer, T. Porwol, U. Berchner-Pfannschmidt, C. Wotzlaw, and H. Acker. Two-photon fluorescence absorption and emission spectra of dyes relevant for cell imaging. J. Microsc. 208:108–115, 2002.
Denk, W., K. R. Delaney, A. Gelperin, D. Kleinfeld, B. W. Strowbridge, D. W. Tank, and R. Yuste. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J. Neurosci. Meth. 54:151–162, 1994.
Denk, W., and P. B. Detwiler. Optical recording of light-evoked calcium signals in the functionally intact retina. Proc. Natl. Acad. Sci. U.S.A. 96:7035–7040, 1999.
Denk, W., and K. Svoboda. Photon upmanship: Why multiphoton imaging is more than a gimmick. Neuron 18:351–357, 1997.
Diaspro, A., and M. Robello. Two-photon excitation of fluorescence for three-dimensional optical imaging of biological structures. J. Photochem. Photobiol. B: Biol. 55:1–8, 2000.
Ding, L., R. Splinter, and S. B. Knisley. Quantifying spatial localization of optical mapping using Monte Carlo simulations. IEEE Trans. Biomed. Eng. 48:1098–1107, 2001.
Efimov, I. R., F. Aguel, Y. Cheng, B. Wollenzier, and N. Trayanova. Virtual electrode polarization in the far field: Implications for external defibrillation. Am. J. Physiol. Heart Circ. Physiol. 279:H1055–H1070, 2000.
Fan, G. Y., H. Fujisaki, A. Miyawaki, R.-K. Tsay, R. Y. Tsien, and M. H. Ellisman. Video-rate scanning two-photon excitation fluorescence microscopy and ratio imaging with cameleons. Biophys. J. 76:2412–2420, 1999.
Hess, S. T., and W. W. Webb. Measurement of fluorescence signal of a voltage-sensitive dye using two-photon excitation. Biophys. J. 74:A201, 1998.
Knisley, S. B. Transmembrane voltage changes during unipolar stimulation of rabbit ventricle. Circ. Res. 77:1229–1239, 1995.
Knisley, S. B., R. K. Justice, W. Kong, and P. L. Johnson. Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. Am. J. Physiol. Heart Circ. Physiol. 279:H1421–H1433, 2000.
Liau, J., J. H. Dumas, D. Janks, B. J. Roth, and S. B. Knisley. Cardiac optical mapping under a translucent stimulation electrode. Ann. Biomed. Eng. 32:1202–1210, 2004.
Mainen, Z. F., M. Maletic-Savatic, S. H. Shi, Y. Hayashi, R. Malinow, and K. Svoboda. Two-photon imaging in living brain slices. Methods. 18:231–239, 1999.
Nygren, A., R. B. Clark, D. D. Belke, C. Kondo, W. R. Giles, and F. X. Witkowski. Voltage-sensitive dye mapping of activation and conduction in adult mouse hearts. Ann. Biomed. Eng. 28:958–967, 2000.
Plonsey, R., and R. C. Barr. Effect of microscopic and macroscopic discontinuities on the response of cardiac tissue to defibrillating (stimulating) currents. Med. Biol. Eng. Comput. 42:130–136, 1986.
Plonsey, R., and R. C. Barr. Electric field stimulation of excitable tissue. IEEE Trans. Biomed. Eng. 42:329–336, 1995.
Ramshesh, V. K., and S. B. Knisley. Spatial localization of cardiac optical mapping with multiphoton excitation. J. Biomed. Opt. 8:253–259, 2003.
Roth, B. J. Artifacts, assumptions, and ambiguity: Pitfalls in comparing experimental results to numerical simulations when studying electrical stimulation of the heart. Chaos 12:973–981, 2002.
Rubart, M., E. Wang, K. W. Dunn, and L. J. Field. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am. J. Physiol. Cell Physiol. 284:C1654–C1668, 2003.
Ruthazer, E. S., and H. T. Cline. Multiphoton imaging of neurons in living tissue: Acquisition and analysis of time-lapse morphological data. Real-Time Imag. 8:175–188, 2002.
Spach, M. S., and M. E. Josephson. Initiating reentry: the role of nonuniform anisotropy in small circuits. J. Cardiovasc. Electrophysiol. 5:182–209, 1994.
Yuste, R., and W. Denk. Dendritic spines as basic functional units of neuronal integration. Nature 375:682–684, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dumas, J.H., Kinisley, S.B. Two-Photon Excitation of di-4-ANEPPS for Optical Recording of Action Potentials in Rabbit Heart. Ann Biomed Eng 33, 1802–1807 (2005). https://doi.org/10.1007/s10439-005-8466-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-8466-9