Abstract
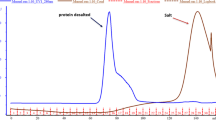
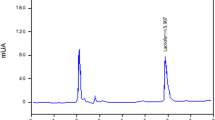
Bovine lactoperoxidase (LPO) was purified with amberlite CG 50 H+ resin, CM sephadex C-50 ion-exchange chromatography, and sephadex G-100 gel filtration chromatography from skim milk. The activity of lactoperoxidase was measured by using 2.2-azino-bis(3-ethylbenzthiazoline-6 sulfonic acid) diammonium salt (ABTS) as a choromogenic substrate at pH 6.0. Purification degree for the purified enzyme was controlled with SDS-PAGE and R z value (A 412/A 280). R z value for the purified LPO was 0.8. K m value at pH 6.0 at 20° C for the LPO was 0.20 mM. V max value was 7.87 μmol/ml min at pH 6.0 at 20°C. Bovine LPO showed high antibacterial activity in 100 mM thiocyanate -100 mM H2O2 medium for some pathogenic bacteria, such as Aeromonas hydrophila ATCC 7966, Micrococcus luteus LA 2971, Mycobacterium smegmatis RUT, Bacillus subtilis IMG 22, Pseudomonas pyocyanea, Bacillus subtilis var. niger ATCC 10, Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 15753, Bacillus brevis FMC3, Klebsiella pneumoniae FMC 5, Corynebacterium xerosis UC 9165, Bacillus cereus EU, Bacillus megaterium NRS, Yersinia enterocolytica, Listeria monocytogenes scoot A, Bacillus megaterium EU, Bacillus megaterium DSM32, Klebsiella oxytocica, Staphylococcus aerogenes, Streptococcus faecalis, Mycobacterium smegmatis CCM 2067 and compared with well known antibacterial substances such as penicilline, ampicilline, amoxicillin-clavulanate and ceftriaxon. The LPO-100 mM thiocyanate-100 mM H2O2 system was purposed as an effective agent against many of the diseases causing organisms in human and animals.
Similar content being viewed by others
REFERENCES
Ueda, T., Sakamaki, K., Kuroki, T., Yano, I., and Nagata, S., Eur. J. Biochem., 1976, vol. 243, pp. 32–41.
Kumar, R. and Bhatla, K.L., Acta Crystallogr., 1995, vol. D51, pp. 1094–1096.
Hamon, C.B. and Klebanoff, S.I., J. Exp. Med., 1973, vol. 134, pp. 438–441.
Golhefors, L. and Marklundi, S., Infect. Immun., 1975, vol. 11, pp. 1210–1215.
Reiter, B. and Perraudin, J.P., in Peroxidase in Chemistry and Biology, Everse, J., Everse, K.E., and Grisham, M.B., Eds., Boca Raton: CRC, 1991, pp. 143–180.
Morrisson, M., Bayse, G., and Danner, D.J., Biochemistry, 1970, vol. 9, pp. 2995–3000.
Cals, M., Maillierat, P., Birignon, G., Anglade, P., and Dumas, B.R., Eur. J. Biochem., 1991, vol. 198, pp. 733–739.
Paul, K.G., Ohlsson, P.I., and Henriksson, A., FEBS Lett., 1980, vol. 110, pp. 200–204.
Dewil, J.N. and Van Hooydonk, A.C.M., Netherlands Milk Dairy, 1996, vol. 50, pp. 227–244.
Caristrom, A., Biochem. Biophys. Acta, 1969, vol. 23, pp. 185–202.
Shin, K., Tomita, M., and Lonnerdal, B., J. Nutr. Biochem, 2000, vol. 11, pp. 95–101.
Morin, D.E., Rowan, L.L., and Hurley, W.L., Small Ruminnat Res., 1995, vol. 17, pp. 255–261.
Elagamy, E.I., Food Chem., 2000, vol. 68, pp. 227–232.
Jacop, B.M., Antony, E., Srekumar, B., and Haridas, M., Life Sci., 2000, vol. 66, pp. 2433–2439.
Jacob, B.M., Manoj, N.K., and Haridas, M., Ind. J. Exp. Biol., 1998, vol. 36, pp. 808–810.
Frank, J.F. and Hassan, A.N., Apply Dairy Microbiology, 1998, vol. 5, pp. 131–172.
Dumonte, C. and Rousst, B., J. Biol. Chem., 1983, vol. 258, pp. 14166–14172.
Ozdemir, H., Aygul, I., and Kufrevioglu, O.I., Preb. Biochem. Biotech., 2001, vol. 31, no.2, pp. 125–134.
Shindler, J.S. and Bardsley, W.G., Biochem. Biophys. Res. Commun., 1975, vol. 67, pp. 1307–1312.
Segel, I.E., Enzyme Kinetics, Toronto: John Wiley and Sons, 1968, p. 403.
Laemmli, D.K., Nature (London), 1970, vol. 227, pp. 680–685.
Haddain, M.S., Ibrahim, S.A., and Robinson, R.K., Food Control, 1996, vol. 7, pp. 149–152.
Kamau, D.N., Doores, S., and Puruitt, K.M., Appl. Environ. Microbiol., 1990, vol. 56, pp. 2711–2716.
Reiter, B., Int. Dairy Feder. Bull., 1985, vol. 191, pp. 2–35.
Aune, T.M. and Thomas, E.L., Eur. J. Biochem., 1977, vol. 80, pp. 209–214.
Ozdemir, H., Hacbeyoglu, H.I., and Uslu, H., Preb. Biochem. Biotechnol., 2002, vol. 32, no.2, pp. 143–155.
Author information
Authors and Affiliations
Additional information
__________
From Prikladnaya Biokhimiya i Mikrobiologiya, Vol. 41, No. 4, 2005, pp. 397–401.
Original English Text Copyright © 2005 by Uguz, Ozdemir.
This article was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Uguz, M.T., Ozdemir, H. Purification of Bovine Milk Lactoperoxidase and Investigation of Antibacterial Properties at Different Thiocyanate Mediated. Appl Biochem Microbiol 41, 349–353 (2005). https://doi.org/10.1007/s10438-005-0059-8
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10438-005-0059-8