Abstract
Background: Management of patients with breast cancers ≤1 cm remains controversial. Reports of infrequent nodal metastases in tumors ≤5 mm has led to suggestions that axillary dissection should be selective, and that tumor characteristics should guide adjuvant therapy.
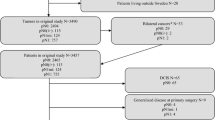
Methods: A retrospective review of 290 patients with breast cancer 1 cm in size or smaller from 1989 to 1991 was done. Distant disease-free survival (DDFS) was the primary outcome measure.
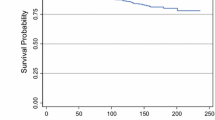
Results: There were 95 T1a (≤5 mm) and 196 T1b (6–10 mm) cancers. Nodal metastases were found in 8 T1a and 26 T1b tumors. Larger size, poorer differentiation, and lymphovascular invasion (LVI) were associated with more nodal metastases, but none of these trends reached statistical significance. The 6-year DDFS was 93% for node-negative and 87% for node-positive patients (P = .02). Overall, breast cancers with poorer differentiation and LVI trended toward a poorer outcome. For patients with node-negative tumors, LVI was associated with a poorer outcome (P = .03). The size of the primary tumor was not predictive of outcome. There were no nodal metastases or recurrences in the 18 patients with microinvasive breast cancer.
Conclusions: Lymph node status is the major determinant of outcome in breast cancers 1 cm in size or smaller. Accurate axillary assessment remains crucial in management of small breast cancer.
Similar content being viewed by others
REFERENCES
NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA 1991;265:391–4.
Silverstein M, Gierson E, Waisman J, Senofsky G, Colburn W, Gamagami P. Axillary lymph node dissection for T1a breast carcinoma: is it indicated? Cancer 1994;73:664–7.
Cady B. Is axillary lymph node dissection necessary in routine management of breast cancer? No. The Breast Journal 1997;3:246–60.
Chontos A, Maher D, Ratzer E, Fenoglio M. Axillary lymph node dissection: is it required in T1a breast cancer? J Am Coll Surg 1997;184:493–8.
Velanovich V. Axillary lymph node dissection for breast cancer: a decision analysis of T1 lesions. Ann Surg Oncol 1998;5:131–9.
Mustafa I, Bland K. Indications for axillary dissection in T1 breast cancer. Ann Surg Oncol 1998;5:4–8.
Walls J, Boggis C, Wilson M, Asbury D, Roberts J, Bundred N, Mansel R. Treatment of the axilla in patients with screen-detected breast cancer. Br J Surg 1993;80:436–8.
Silverstein M, Gierson E, Waisman J, Colburn W, Gamagami P. Predicting axillary node positivity in patients with invasive carcinoma of the breast by using a combination of T category and palpability. J Am Coll Surg 1995;180:700–7004.
Dowlatshahi K, Snider H, Kim R. Axillary node status in nonpalpable breast cancer. Ann Surg Oncol 1995;2:424–428.
Barth A, Craig P, Silverstein M. Predictors of axillary lymph node metastases in patients with T1 breast cancer. Cancer 1997;79:1918–1922.
Carter C, Allen C, Henson D. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989; 63:181–187.
Giuliano A, Barth A, Spivack B, Beitsch PD, Evans SW. Incidence, and predictors of axillary metastasis in T1 carcinoma of the breast. J Am Coll Surg 1996;183:185–189.
Shetty M, Reiman H Jr. Tumor size and axillary metastasis, a correlative occurrence in 1244 cases of breast cancer between 1980 and 1995. Eur J Surg Oncol 1997;23:139–141.
White R, Vezeridis M, Konstadoulakis M, Cole B, Wanebo H, Bland K. Therapeutic options and results for the management of minimally invasive carcinoma of the breast: influence of axillary dissection for treatment of T1a and T1b lesions. J Am Coll Surg 1996;183:575–582.
Rush Port E, Tan L, Borgen P, Van Zee K. Incidence of axillary lymph node metastases in T1a and T1b breast carcinoma. Ann Surg Oncol 1998;5:23–27.
Robinson D, Senofsky G, Ketcham A. Role, and extent of lymphadenectomy for early breast cancer. Semin Surg Oncol 1992;8:78–82.
Morrow M. Role of axillary dissection in breast cancer management. Ann Surg Oncol 1996;3:233–234.
Shetty M. Axillary lymph node metastasis in carcinoma of the breast. J Am Coll Surg 1997;184:671–672.
Baxter N, McCready D, Chapman J, et al. Clinical behavior of untreated axillary nodes after local treatment for primary breast cancer. Ann Surg Oncol 1996;3:235–240.
Sosa J, Diener-West M, Gusev Y, Choti M, Lange J, Dooley W, Zeiger M. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol 1998;5:140–149.
Giuliano A, Jones R, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol 1997;15:2345–2350.
Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997;349:1864–1867.
Kiricuta C, Tausch J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 1992;69:2496–2501.
Chadha M, Chabon A, Friedmann P, Vikram B. Predictors of axillary lymph node metastases in patients with T1 breast cancer. Cancer 1993;73:350–353.
Fein D, Fowble B, Hanlon A, et al. Identification of women with T1–T2 breast cancer at low risk of positive axillary nodes. J surg Oncol 1997;65:34–39.
Olivotto I, Jackson J, Mates D, Anderson S, Davidson W, Bryce C, Ragaz J. Prediction of axillary lymph node involvement of women with invasive breast carcinoma: a multivariate analysis. Cancer 1998;83:948–955.
Leitner S, Swern A, Weinberger D, Duncan L, Hutter R. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0). Cancer 1995;76:2266–2274.
International Breast Cancer Study Group. Prognostic importance of occult axillary lymph node micrometastases from breast cancers. Lancet 1990;335:1565–1568.
Kinne D, Petrek J, Osborne M, Fracchia A, DePalo A, Rosen P. Breast carcinoma in situ. Arch Surg 1989;124:33–36.
Wong J, Kopald K, Morton D. The impact of microinvasion on axillary node metastases and survival in patients with intraductal breast cancer. Arch Surg 1990;125:1298–1302.
Silverstein M, Waisman J, Gamagami P, et al. Intraductal carcinoma of the breast (208 cases): clinical factors influencing treatment choice. Cancer 1990;66:102–108.
Solin L, Fowble B, Yeh I, Kowalyshyn M, Schultz D, Weiss M, Goodman R. Microinvasive ductal carcinoma of the breast treated with breast-conserving surgery and definitive irradiation. Int J Radiat Oncol Biol Phys 1992;23:961–968.
Harris JR, Osteen RT. Patients with early breast cancer benefit from effective axillary treatment. Breast Cancer Res Treat 1985; 5:17–21.
Hayward J, Caleffi M. The significance of local control in the primary treatment of breast cancer. Arch Surg 1987;122:1244–1247.
Ruffin WK, Stacey-Clear A, Younger J, Hoover HC Jr. Rationale for routine axillary dissection in carcinoma of the breast. J Am Coll Surg 1995;180:245–251.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mann, G.B., Port, E.R., Rizza, C. et al. Six-Year Follow-Up of Patients With Microinvasive, T1a, and T1b Breast Carcinoma. Ann Surg Oncol 6, 591–598 (1999). https://doi.org/10.1007/s10434-999-0591-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-999-0591-5