Abstract
Background Our aim was to ascertain whether or not the response to preoperative chemoradiotherapy for rectal cancer is associated with p27kip1 and p53 protein expression.
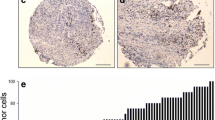
Methods: Thirty-eight patients (27 male, 11 female) with a mean age of 59 years (age range 33–87) and stage II-III rectal cancer received preoperative chemoradiotherapy (45–50.4 Gy; 5-FU 350 mg/m2/day and leucovorin 10 mg/m2/day). Thirty-one underwent low anterior resection; seven underwent abdominoperineal excision. Endoscopic tumor biopsies, performed before adjuvant therapy, were evaluated for: histologic type, tumor differentiation, mitotic index, and p27kip1 and p53 protein expression which were immunohistochemically determined. p53 expression was graded as: a) absent or present in ≤10% of tumor cells; b) present in 11–25%; c) present in 26–75%; and d) present in >75% of tumor cells. p27kip1 expression was assessed using both light microscopy (percent of stained cells x10 HPF) and cytometry with an image analysis workstation. Tumor response, ascertained with histology, was classified using a scale from 0 (no response) to 6 (complete pathologic response).
Results: The mitotic index for the endoscopic biopsies was low in 14 cases, moderate in 17 cases, and high in 7 cases. p53 protein expression was found in 21 (a), 3 (b), 3 (c), and 11 (d) cases. The mean percentage of cells expressing the p27kip1 protein was 34 (range 0–77.14%). A close correlation was found between cytometric and light microscopy findings for p27kip1 (r2 = 0.92, P = .0001). Tumor differentiation was good in 5 cases, poor in 2 cases, and moderate in the remaining 31 cases. While the response to adjuvant therapy was good/complete in 25 (65.78%) cases, it was absent/poor in 13 (34.21%) cases. Univariate analysis associated type of adjuvant therapy (chemoradiotherapy, P = .0428) and p27kip1 protein lower expression (P = .0148) with a poor response to adjuvant treatment. Stepwise linear regression found overexpression of p53 and p27kip1 and young age to be independent variables that were linked to a good response to adjuvant therapy.
Conclusions:Lack of p27kip1 and p53 protein expression in rectal cancer is associated with a poor response to preoperative adjuvant therapy.
Similar content being viewed by others
References
National Cancer Institute statisticians, SEER Cancer Statistics Review, 1973-1995. Bethesda, MD: National Cancer Institute, 1998.
Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995; 221: 778–86.
Gerard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg 1988; 208: 606–14.
Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988; 80: 21–9.
Cedemark B. The Stockholm II trial on preoperative short-term radiotherapy in operable rectal carcinoma: a prospective randomized trial. Proc Am Soc Clin Oncol 1994; 13: 198–201.
Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980–7.
Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–15.
Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1985; 122: 321–6.
Boulis-Wassif S, Gerard A, Loygue J, Camelot D, Buyse M, Duez N. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone or in combination with 5-fluorouracil, followed by radical surgery. Trial of the European Organization on Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Cancer 1984; 53: 1811–8.
Minsky B, Cohen A, Enker W, et al. Preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for rectal cancer. Cancer 1994; 73: 273–80.
Grann A, Minsky BD, Cohen AM, et al. Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 1997; 40: 515–22.
Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum 1997; 40: 131–9.
Mohiuddin M, Regine WF, Marks GJ, Marks JW. High-dose preoperative radiation and the challenge of sphincter-preservation surgery for cancer of the distal 2 cm of the rectum. Int J Radiat Oncol Biol Phys 1998; 40: 569–74.
Meade PG, Blatchford GJ, Thorson AG, Christensen MA, Ternent CA. Preoperative chemoradiation downstages locally advanced ultrasound-staged rectal cancer. Am J Surg 1995; 170: 609–12.
Berard P, Papillon J. Role of pre-operative irradiation for anal preservation in cancer of the low rectum. World J Surg 1992; 16: 502–9.
Rouanet P, Fabre JM, Dubois JB, et al. Conservative surgery for low rectal carcinoma after high-dose radiation. Ann Surg 1995; 221: 67–73.
Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys 1997; 37: 619–27.
Forrett-Kaminsky MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys 1998; 42: 935–41.
Staunton MJ, Gaffney EF. Apoptosis: basic concepts and potential significance in human cancer. Arch Pathol Lab Med 1998; 122: 310–9.
Martin SJ, Green DR. Apoptosis as a goal of cancer therapy. Curr Opin Oncol 1994; 6: 616–21.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993; 74: 957–67.
Scott N, Hale A, Deakin M, et al. A histopathological assessment of the response of rectal adenocarcinoma to combination chemo-radiotherapy: relationship to apoptotic activity, p53 and bcl-2 expression. Eur J Surg Oncol 1998; 24: 169–73.
Hong SI, Hong WS, Jang JJ, et al. Alterations of p53 gene in primary gastric cancer tissues. Anticancer Res 1994; 14: 1251–5.
Scarpa A, Capelli P, Mukai K, et al. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol 1993; 142: 1534–43.
Bertorelle R, Esposito G, Del Mistro A, et al. Association of p53 gene and protein alterations with metastases in colorectal cancer. Am J Surg Pathol 1995; 19: 463–71.
Yamaguchi A, Kurosaka Y, Fushida S, Kanno M, Yonemura Y, Miwa K, Miyazaki I. Expression of p53 protein in colorectal cancer and its relationship to short-term prognosis. Cancer 1992; 70: 2778–84.
Patel DD, Bhatavdekar JM, Chikhlikar PR, et al. Node negative breast carcinoma: hyperprolactinemia and/or overexpression of p53 as an independent predictor of poor prognosis compared to newer and established prognosticators. J Surg Oncol 1996; 62: 86–92.
Cordon-Cardo C, Sheinfeld J, Dalbagni G. Genetic studies and molecular markers of bladder cancer. Semin Surg Oncol 1997; 13: 319–27.
Bell SM, Scott N, Cross D, et al. Prognostic value of p53 overexpression and c-Ki-ras gene mutations in colorectal cancer. Gastroenterology 1993; 104: 57–64.
Poller DN, Baxter KJ, Shepherd NA. p53 and Rb1 protein expression: are they prognostically useful in colorectal cancer?. Br J Cancer 1997; 75: 87–93.
Ahnen DJ, Feigl P, Quan G, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 1998; 58: 1149–58.
Belluco C, Guillem JG, Kemeny N, et al. p53 nuclear protein overexpression in colorectal cancer: a dominant predictor of survival in patients with advanced hepatic metastases. J Clin Oncol 1996; 14: 2696–701.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991; 51: 6304–11.
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A 1992; 89: 7491–5.
Clarke AR, Purdie CA, Harrison DJ, et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 1993; 362: 849–52.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993; 362: 847–9.
Polyak K, Lee MH, Erdjument-Bromage H, et al. Cloning of p27kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994; 78: 59–66.
Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994; 78: 67–74.
Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol 1994; 14: 3683–94.
Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995; 9: 1149–63.
Sherr CJ. Cancer cell cycles. Science 1996; 274: 1672–7.
Fero ML, Rivkin M, Tasch M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 1996; 85: 733–44.
Durand B, Gao FB, Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J 1997; 16: 306–17.
St. Croix B, Florenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS. Impact of the cyclin-dependent kinase inhibitor p27kip1 on resistance of tumor cells to anticancer agents. Nat med 1996; 2: 1204–10.
Katayose Y, Kim M, Rakkar AN, LiZ, Cowan KH, Seth P. Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res 1997; 57: 5441–5.
AJCC American Joint Committee on Cancer. Colon and Rectum. In: Fleming ID, Cooper JS, Henson DE, et al, eds. AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott-Raven, 1997:83–90.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997; 12: 19–23.
Pucciarelli S, Friso ML, Toppan P, et al. Preoperative combined radiotherapy and chemotherapy for middle and lower rectal cancer: preliminary results. Ann Surg Oncol 2000; 7: 38–44.
Wang X, Gorospe M, Huang Y, Holbrook NJ. p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene 1997; 15: 2991–7.
Schreiber M, Muller WJ, Singh G, Graham FL. Comparison of the effectiveness of adenovirus vectors expressing cyclin kinase inhibitors p16 INK4A, p18INK4C, p19INK4D, p21WAF1/CIP1 and p27KIP1 in inducing cell cycle arrest, apoptosis and inhibition of tumorigenicity. Oncogene 1999; 18: 1663–76.
Eymin B, Haugg M, Droin N, Sordet O, Dimanche-Boitrel MT, Solary E. p27Kip1 induces drug resistance by preventing apoptosis upstream of cytochrome c release and procaspase-3 activation in leukemic cells. Oncogene 1999; 18: 1411–8.
Fujieda S, Inuzuka M, Tanaka N, et al. Expression of p27 is associated with Bax expression and spontaneous apoptosis in oral and oropharyngeal carcinoma. Int J Cancer 1999; 84: 315–20.
Ohtani M, Isozaki H, Fujii K, et al. Impact of the expression of cyclin-dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer 1999; 85: 1711–8.
WuJ, Shen ZZ, LuJS, et al. Prognostic role of p27Kip1 and apoptosis in human breast cancer. Br J Cancer 1999; 79: 1572–8.
Kawamata N, Morosetti R, Miller CW, et al. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 1995; 55: 2266–9.
Pagano M, Tam SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995; 269: 682–5.
Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science 1996; 271: 1861–4.
Brett MC, Pickard M, Green B, et al. p53 protein overexpression and response to biomodulated 5-fluorouracil chemotherapy in patients with advanced colorectal cancer. Eur J Surg Oncol 1996; 22: 182–5.
Tannapfel A, NüssleinS, Fietkau R, Katalinic A, KöckerlingF, Wittekind C. Apoptosis, proliferation, bax, bcl-2 and p53 status prior to and after preoperative radiochemotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 1998; 41: 585–91.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992; 69: 1237–45.
Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature 1991; 351: 453–6.
Bissonnette N, Wasylyk B, Hunting DJ. The apoptotic and transcriptional transactivation activities of p53 can be dissociated. Biochem Cell Biol 1997; 75: 351–8.
Gullouf C, Rosselli F, Sjin RT, Moustacchi E, Hoffman B, Liebermann DA. Role of a mutant p53 protein in apoptosis: characterization of a function independent of transcriptional trans-activation. Int J Oncol 1998; 13: 107–14.
Saller E, Tom E, Brunori M, Otter M, Estreicher A, Mack DH, Iggo R. Increased apoptosis induction by 121F mutant p53. EMBO J 1999; 18: 4424–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esposito, G., Pucciarelli, S., Alaggio, R. et al. p27kip1 Expression Is Associated With Tumor Response to Preoperative Chemoradiotherapy in Rectal Cancer. Ann Surg Oncol 8, 311–318 (2001). https://doi.org/10.1007/s10434-001-0311-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-001-0311-2