Abstract
Electrode stress is one of the main driving forces of electrochemical degradation, which is directly related to battery cycle life, thus attracting great interest. Herein, we propose an in situ method to measure bilayer stresses in film-substrate electrodes during electrochemical processes. This method consists of two parts: stress models featuring Li-dependent material modulus and in situ deformation measurements, through which electrode bilayer stresses evolution accompanied by Li-dependent material modulus can be quantitatively characterized. As application of the method, typical silicon-composite and carbon-composite film-substrate electrodes are selected for in situ mechanical measurements and experimental analysis is performed. Results show that silicon material and carbon material exhibit significant, continuous softening and stiffening, respectively. In two film-substrate electrodes, electrode material films experience compressive stress and current collector substrates undergo a tensile-to-compressive conversion across the thickness. Besides, moduli and stresses in both electrodes vary nonlinearly with capacity, presenting non-overlapping paths between lithiation and delithiation. Based on experimental data, we further demonstrate the key role of Li-dependent modulus on electrode stresses, finding that silicon material softening decreases and carbon material stiffening increases electrode stresses. The deficiencies of current stress measurement method based on Stoney equation and the applicability of our method are discussed.
Graphic Abstract









Similar content being viewed by others
References
Hofmann, T., Westhoff, D., Feinauer, J., et al.: Electro-chemo-mechanical simulation for lithium ion batteries across the scales. Int. J. Solids Struct. 184, 24–39 (2020)
Zhao, Y., Stein, P., Bai, Y., et al.: A review on modeling of electro-chemo-mechanics in lithium-ion batteries. J. Power Sources 413, 259–283 (2019)
Zhang, Y., Guo, Z.: Numerical computation of central crack growth in an active particle of electrodes influenced by multiple factors. Acta. Mech. Sin. 34, 706–715 (2018)
Palacin, M.R., de Guibert, A.: Why do batteries fail? Science 351, 1253292 (2016)
Ebner, M., Marone, F., Stampanoni, M., et al.: Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science 342, 716–720 (2013)
Liu, M., Lu, B., Shi, D.L., et al.: Two-dimensional analysis of progressive delamination in thin film electrodes. Acta. Mech. Sin. 34, 359–370 (2018)
Li, Y., Mao, W., Zhang, K., et al.: Analysis of large-deformed electrode of lithium-ion battery: effects of defect evolution and solid reaction. Int. J. Solids Struct. 170, 1–10 (2019)
Xie, H.M., Guo, J.G., Song, H.B., et al.: Active tensile/compressive stress-regulated electrochemical behavior and mechanism in electrodes. Energy Technol. 6, 1788–1796 (2018)
Yang, W., Xie, H., Shi, B., et al.: In-situ experimental measurements of lithium concentration distribution and strain field of graphite electrodes during electrochemical process. J. Power. Sources. 423, 174–182 (2019)
Suo, Y., Yang, F.: One-dimensional analysis of the coupling between diffusion and deformation in a bilayer electrode. Acta. Mech. Sin. 35, 589–599 (2018)
Cui, Z., Gao, F., Cui, Z., et al.: A second nearest-neighbor embedded atom method interatomic potential for Li–Si alloys. J. Power. Sources. 207, 150–159 (2012)
Cheng, Y.T., Verbrugge, M.W.: Evolution of stress within a spherical insertion electrode particle under potentiostatic and galvanostatic operation. J. Power. Sources. 190, 453–460 (2009)
Yang, H., Huang, S., Huang, X., et al.: Orientation-dependent interfacial mobility governs the anisotropic swelling in lithiated silicon nanowires. Nano. Lett. 12, 1953–1958 (2012)
Zhao, K.J., Pharr, M., Hartle, L., et al.: Fracture and debonding in lithium-ion batteries with electrodes of hollow core-shell nanostructures. J. Power. Sources. 218, 6–14 (2012)
Hao, F., Fang, D.: Diffusion-induced stresses of spherical core-shell electrodes in lithium-ion batteries: the effects of the shell and surface/interface stress. J. Electrochem. Soc. 160, A595–A600 (2013)
Zang, J.L., Zhao, Y.P.: A diffusion and curvature dependent surface elastic model with application to stress analysis of anode in lithium ion battery. Int. J. Eng. Sci. 61, 156–170 (2012)
Chew, H.B., Hou, B., Wang, X., et al.: Cracking mechanisms in lithiated silicon thin film electrodes. Int. J. Solids Struct. 51, 4176–4187 (2014)
Lu, B., Song, Y., Guo, Z., et al.: Modeling of progressive delamination in a thin film driven by diffusion-induced stresses. Int. J. Solids Struct. 50, 2495–2507 (2013)
Ji, L., Guo, Z.: Analytical modeling and simulation of porous electrodes: Li-ion distribution and diffusion-induced stress. Acta. Mech. Sin. 34, 187–198 (2017)
Deshpande, R., Cheng, Y.T., Verbrugge, M.W.: Modeling diffusion-induced stress in nanowire electrode structures. J. Power. Sources. 195, 5081–5088 (2010)
Zhang, J., Lu, B., Song, Y., et al.: Diffusion induced stress in layered Li-ion battery electrode plates. J. Power. Sources. 209, 220–227 (2012)
Song, Y., Shao, X., Guo, Z., et al.: Role of material properties and mechanical constraint on stress-assisted diffusion in plate electrodes of lithium ion batteries. J. Phys. D Appl. Phys. 46, 105307 (2013)
Lu, B., Zhao, Y., Song, Y., et al.: Stress-limited fast charging methods with time-varying current in lithium-ion batteries. Electrochim. Acta 288, 144–152 (2018)
Chang, L., Lu, Y., He, L., et al.: Phase field model for two-phase lithiation in an arbitrarily shaped elastoplastic electrode particle under galvanostatic and potentiostatic operations. Int. J. Solids Struct. 143, 73–83 (2018)
Shenoy, V.B., Johari, P., Qi, Y.: Elastic softening of amorphous and crystalline Li–Si phases with increasing Li concentration: a first-principles study. J. Power. Sources. 195, 6825–6830 (2010)
Qi, Y., Guo, H., Hector, L.G., et al.: Threefold increase in the Young’s modulus of graphite negative electrode during lithium intercalation. J. Electrochem. Soc. 157, A558 (2010)
Qi, Y., Hector, L.G., James, C., et al.: Lithium concentration dependent elastic properties of battery electrode materials from first principles calculations. J. Electrochem. Soc. 161, F3010–F3018 (2014)
Verma, A., Kotaka, T., Tabuchi, Y., et al.: Mechano-electrochemical interaction and degradation in graphite electrode with surface film. J. Electrochem. Soc. 165, A2397–A2408 (2018)
Lu, Y., Zhang, P., Wang, F., et al.: Reaction-diffusion-stress coupling model for Li-ion batteries: the role of surface effects on electrochemical performance. Electrochim. Acta 274, 359–369 (2018)
He, Y.L., Hu, H.J., Song, Y.C., et al.: Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power. Sources. 248, 517–523 (2014)
Zhou, W., Hao, F., Fang, D.: The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 05, 1350040 (2013)
Wen, J., Wei, Y., Cheng, Y.T.: Examining the validity of Stoney-equation for in situ stress measurements in thin film electrodes using a large-deformation finite-element procedure. J. Power. Sources. 387, 126–134 (2018)
Xie, H., Kang, Y., Song, H., et al.: Modified stoney model and optimization of electrode structure based on stress characteristics. Energy Technol. 7, 333–345 (2019)
Qiu, W., Cheng, C.L., Liang, R.R., et al.: Measurement of residual stress in a multi-layer semiconductor heterostructure by micro-Raman spectroscopy. Acta. Mech. Sin. 32, 805–812 (2016)
Xu, C., Xue, T., Qiu, W., et al.: Size effect of the interfacial mechanical behavior of graphene on a stretchable substrate. ACS Appl. Mater. Interfaces. 8, 27099–27106 (2016)
Yu, L., Pan, B.: Single-camera stereo-digital image correlation with a four-mirror adapter: optimized design and validation. Opt. Laser Eng. 87, 120–128 (2016)
Song, H., Zhang, H., Fu, D., et al.: Experimental analysis and characterization of damage evolution in rock under cyclic loading. Int. J. Rock Mech. Min. Sci. 88, 157–164 (2016)
Wu, L., Cheng, T., Zhang, Q.C.: A bi-material microcantilever temperature sensor based on optical readout. Measurement 45, 1801–1806 (2012)
Cheng, X.M., Pecht, M.: In situ stress measurement techniques on Li-ion battery electrodes: a review. Energies 10, 591 (2017)
Jangid, M.K., Mukhopadhyay, A.: Real-time monitoring of stress development during electrochemical cycling of electrode materials for Li-ion batteries: overview and perspectives. J. Mater. Chem. A 7, 23679–23726 (2019)
Yang, L., Chen, H.S., Song, W.L., et al.: In situ optical observations and simulations on defect induced failure of silicon island anodes. J. Power. Sources. 405, 101–105 (2018)
Wang, Z., Huang, H., Zeng, L., et al.: In-operando deformation studies on the mechano-electrochemical mechanism in free-standing MWCNTs/V2O5 lithium ion battery electrode. Electrochim. Acta 305, 101–115 (2019)
Pharr, M., Suo, Z., Vlassak, J.J.: Variation of stress with charging rate due to strain-rate sensitivity of silicon electrodes of Li-ion batteries. J. Power. Sources. 270, 569–575 (2014)
Nadimpalli, S.P.V., Sethuraman, V.A., Bucci, G., et al.: On plastic deformation and fracture in Si films during electrochemical lithiation/delithiation cycling. J. Electrochem. Soc. 160, A1885–A1893 (2013)
Soni, S.K., Sheldon, B.W., Xiao, X., et al.: Stress mitigation during the lithiation of patterned amorphous Si islands. J. Electrochem. Soc. 159, A38 (2012)
Mukhopadhyay, A., Tokranov, A., Sena, K., et al.: Thin film graphite electrodes with low stress generation during Li-intercalation. Carbon 49, 2742–2749 (2011)
Sonia, F.J., Ananthoju, B., Jangid, M.K., et al.: Insight into the mechanical integrity of few-layers graphene upon lithiation/delithiation via in situ monitoring of stress development. Carbon 88, 206–214 (2015)
Sethuraman, V.A., van Winkle, N., Abraham, D.P., et al.: Real-time stress measurements in lithium-ion battery negative-electrodes. J. Power. Sources. 206, 334–342 (2012)
Sethuraman, V.A., Nguyen, A., Chon, M.J., et al.: Stress evolution in composite silicon electrodes during lithiation/delithiation. J. Electrochem. Soc. 160, A739–A746 (2013)
Kumar, R., Woo, J.H., Xiao, X., et al.: Internal microstructural changes and stress evolution in silicon nanoparticle based composite electrodes. J. Electrochem. Soc. 164, A3750–A3765 (2017)
Li, D., Wang, Y., Hu, J., et al.: Role of polymeric binders on mechanical behavior and cracking resistance of silicon composite electrodes during electrochemical cycling. J. Power. Sources. 387, 9–15 (2018)
Xie, H., Zhang, Q., Song, H., et al.: Modeling and in situ characterization of lithiation-induced stress in electrodes during the coupled mechano-electro-chemical process. J. Power. Sources. 342, 896–903 (2017)
Xie, H.M., Kang, Y.L., Song, H.B., et al.: Real-time measurements and experimental analysis of material softening and total stresses of Si-composite electrode. J. Power. Sources. 424, 100–107 (2019)
Yang, B., He, Y.P., Irsa, J., et al.: Effects of composition-dependent modulus, finite concentration and boundary constraint on Li-ion diffusion and stresses in a bilayer Cu-coated Si nano-anode. J. Power. Sources. 204, 168–176 (2012)
Xie, H., Song, H., Kang, Y., et al.: In situ experimental measurement of the mechanical properties of carbon-based electrodes during the electrochemical process. J. Electrochem. Soc. 165, A2069–A2074 (2018)
Freund, L.B., Suresh, S.: Thin film materials: stress, Defect Formation and Surface Evolution. Cambridge University Press, Cambridge (2004)
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grants 11890680 and 11672203 and 12022205).
Author information
Authors and Affiliations
Corresponding authors
Appendices
Appendix 1: Experimental details
1.1 Electrode preparation
The preparation process of bilayer electrodes was described in our previous work [52, 53, 55]. Cu foil was selected as the current collector substrate. It was cut into strips of ~ 25 mm length and ~ 4 mm width to measure deformation, presenting a thickness of 28 μm (20 μm) in the silicon-composite (carbon-composite) film-substrate electrodes. The electrode material film of silicon-composite electrode was a mixture of crystalline silicon nanoparticles (~ 80 nm diameter, 99% purity, Guangzhou Hongwu, China), sodium alginate binder (99.5%, 18th Research Institute, Tianjin, China) and conductive additive Super p (99.5%, Timcal, Switzerland) in a mass ratio of 70:15:15, giving a 18 μm thickness. The electrode material film of carbon-composite electrode was a mixture of mesocarbon microbead particulates (MCMB, 10-20 μm, 99% purity, 18th Research Institute, Tianjin, China), polyvinylidene fluoride binder (PVDF, 99.5%, 18th Research Institute, Tianjin, China) and conductive additive Super p in a mass ratio of 90:5:5, giving a 10 μm thickness.
1.2 Cell assembly
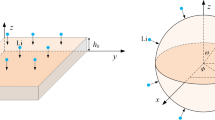
A home-made electrochemical cell that featured a glass window for in situ deformation measurements was assembled in an argon-filled glove-box with an electrolyte solution of 1 M lithium hexafluorophosphate (LiPF6) in a 1:1 volume ratio of ethylene carbonate:dimethyl carbonate (99.5%, Tianjin Jinniu, China). As illustrated schematically in Fig. 3, the film-substrate electrode served as the working electrode within the in situ cell, with a lithium foil (~ 120 μm thickness, 99.9% purity, Tianjin Zhongneng, China) as the reference/counter electrode. The two electrodes, in the form of two cantilever beams featuring a fixed upper end and a free lower end, were separated by a microporous polypropylene film (~ 28 μm thickness, Celgard Inc., USA) to prevent physical contact and were positioned facing each other, with the lateral side parallel to the glass window, thereby allowing direct in situ deformation measurements through the glass window.
1.3 Electrochemical test
Electrochemical experiments were performed in the electrochemical cell at room temperature using a M210A cell tester (Wuhan LAND, China). The two composite film-substrate electrodes were lithiated and delithiated between a potential range of 2 V and 0.01 V vs. Li/Li+ at a constant current of C/7 (i.e., 7 h to lithiation and 7 h to delithiation), where C denotes the theoretical specific capacity of active material, having values of 3579 mAh g−1 for silicon and 372 mAh g−1 for carbon [2, 28, 39, 50, 51].
Appendix 2: Experimental parameters
2.1 Li concentration \(\bar{c}\)
Assuming macroscopically spatially uniform lithium ions, the Li concentration can be approximately obtained by its normalized capacity using the expression of \(\bar{c}\)= c/C, where c is the real-time capacity measured by a cell tester, as shown in Fig. 4.
2.2 Chemical expansion coefficient β
β, an intrinsic property of an electrode material film that depends on its composition and structure, can be determined by maximum expansion of the electrode material film, Ve-max, in two steps [52, 53, 55]. In the first step, in a composite electrode material film comprising active particles and inactive components, such as binder, conductive additives, and pores, Ve-max can be obtained by superimposing the maximum expansion of active particles (ηVp-max) and volume of all inactive components (1 − η), expressed as Ve-max = ηVp-max+ (1 − η), where Vp-max represents the maximum expansion of active particles and it is assumed a constant volume of inactive components. In the second step, in analogy with thermal expansion and assuming that expansion of the electrode material film is proportional to the Li concentration, Ve-max can be also formulated as (1 + β)3. Therefore, β was calculated using the expression β = [(ηVp-max + 1 − η)1/3 −1] to be 0.33 for the silicon-composite film-substrate electrode with Ve-max = 235% and 0.023 for the carbon-composite film-substrate electrode with Ve-max = 107%. By comparison, the chemical expansion coefficients obtained here were close to existing values that calculated by Ωcmaxη/3 with consideration of volume fraction of active particles, in which Ω and cmax are partial molar volume and maximum molar Li-ion concentration of active particles [30, 31, 51].
2.3 Thickness h e
The thickness of the electrode material film changes with Li concentration and can be obtained from expansion of the electrode material film using the expression he = h 0e [1 + (Ve-max − 1)\(\bar{c}\)], where h 0e is the initial thickness. This expression assumes that expansion is totally accommodated by growth in the thickness direction owing to the in-plane restriction by the Cu substrate, and considers the linear relationship between thickness growth and Li concentration that appears in most studies [43,44,45, 50]. Therefore, the Li concentration-dependent thickness expressions in the silicon-composite and carbon-composite film-substrate electrodes were \(h_{e}^{0} (1 + 1.35\bar{c})\) and \(h_{e}^{0} (1 + 0.07\bar{c})\), respectively.
Appendix 3: Error analysis of simplified stress model
Substituting the parameters in Table 1 and experimental results of Figs. 4, 5 into the initial stress model (Eqs. (10) and (11)) and simplified stress model (Eqs. (13) and (14)), the stresses were calculated for two models, as shown in Fig. 9 for the silicon-composite and carbon-composite film-substrate electrodes. In both film-substrate electrodes, the stress evolution of the simplified model was always consistent with that of the initial model. Values obtained from the simplified model were slightly larger by no more than 2% for two composite film-substrate electrodes. Such experimental data demonstrated that the simplified model could perfectly characterize stress evolution of two composite film-substrate electrodes, as expounded in Sect. 3.
Bilayer stress as a function of capacity in a, c, e silicon-composite and b, d, f carbon-composite film-substrate electrodes, calculated from simplified stress model (Eqs. (13) and (14)) and initial stress model (Eqs. (10) and (11)): a, b stress of electrode material film; c, d interface stress of current collector substrate; e, f surface stress of current collector substrate
Appendix 4: Quantitative relationship between stress and Li-dependent modulus
Variable degrees were defined: for modulus, it is (Ee-Si-softening − Ee0-Si)/Ee0-Si and (Ee-C-stiffening − Ee0-C)/Ee0-C in silicon-composite and carbon-composite film-substrate electrodes, respectively and the stress corresponding to modulus was used to define the stress variable degree. Therefore, negative values represent softening and stress decrease, while positive values are the opposite. Then, based on the experimental data shown in Figs. 5, 7 and 10 shows the evolution of variable degree in material modulus and stresses with capacity during the second lithiation for silicon-composite and carbon-composite film-substrate electrodes. The results show that in the two electrodes, variable degree of silicon softening (carbon stiffening) was consistent with that of stress decrease (increase), presenting almost the same values at any lithiation state. Taking lithiation end as example: in silicon electrode, surface/interface stresses decreased by about 87.9%/88.9%/88.4%/88.3% associated with a 89.5% decrease in modulus; in carbon electrode, surface/interface stresses increased by about 95.7%/96%/95.8%/95.8% associated with a 96.5% increase in modulus. These data emphasize the importance of a Li-dependent material modulus for stress measurement.
Rights and permissions
About this article
Cite this article
Xie, H., Kang, Y., Song, H. et al. In situ method for stress measurements in film-substrate electrodes during electrochemical processes: key role of softening and stiffening. Acta Mech. Sin. 36, 1319–1335 (2020). https://doi.org/10.1007/s10409-020-00995-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-020-00995-8