Abstract
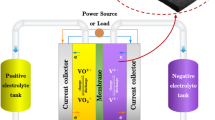
In this work, we present a comprehensive study dealing with the modeling of the conversion process occurring in a redox flow cell. Experiments are carried out on an original millifluidic flow battery with ferrocyanide and iodide as electrolytes. A simulation model supports the experimental data. In flow, intensity recovery is limited by the mass transfer. Thanks to diffusion, at low Peclet, the conversion is complete. On the contrary, at high Peclet, the convection prevents the diffusion of species and induces a conversion drop. A quantitative agreement is found between theoretic model and experiment both on current and on power curves. The originality of our work is to take into account the kinetics of the redox reaction at the electrodes. We evidence a new regime where the current intensity is constant as a function of the Peclet number. The maximal recovered power is obtained at a given flow rate and not at very high flow rate. This work paves the road for the optimization of the conversion process and for the measurements of the thermodynamic parameters involved in the redox process such as kinetic parameters at the electrodes.








Similar content being viewed by others
References
Alotto P, Guarnieri M, Moro F (2014) Redox flow batteries for the storage of renewable energy: a review. Renew Sustain Energy Rev 29:325–335
Bazylak A, Sinton D, Djilali N (2005) Improved fuel utilization in microfluidic fuel cells: a computational study. J Power Sources 143(1–2):57–66
Booth J, Compton RG, Cooper JA, Dryfe RA, Fisher AC, Davies CL, Walters MK (1995) Hydrodynamic voltammetry with channel electrodes: microdisk electrodes. J Phys Chem 99(27):10942–10947
Chang M-H, Chen F, Fang N-S (2006) Analysis of membraneless fuel cell using laminar flow in a Y-shaped microchannel. J Power Sources 159(2):810–816
Choban ER, Waszczuk P, Kenis PJA (2005) Characterization of limiting factors in laminar flow-based membraneless microfuel cells. Electrochem Solid State Lett 8(7):A348
El-Hallag IS (2010) Electrochemical oxidation of iodide at a glassy carbon electrode in methylene chloride at various temperatures J Chil Chem Soc 55(1):67–73
Gabe DB (1974) The rotating cylinder electrode. J Appl Electrochem 4:91–108
Kjeang E, Michel R, Harrington DA, Djilali N, Sinton D (2008) A microfluidic fuel cell with flow-through porous electrodes. J Am Chem Soc 130(12):4000–4006
Kjeang E, Proctor BT, Brolo AG, Harrington DA, Djilali N, Sinton D (2007) High-performance microfluidic vanadium redox fuel cell. Electrochim Acta 52(15):4942–4946
Kjeang E, Roesch B, McKechnie J, Harrington DA, Djilali N, Sinton D (2007) Integrated electrochemical velocimetry for microfluidic devices. Microfluid Nanofluidics 3(4):403–416
Leung P, Li X, de Leon CP, Berlouis L, Low CTJ, Walsh FC (2012) Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Advances 2(27):10125
Lide DR (2003) Handbook of Chemistry and Physics, 84e edn. CRC Press, Boca Raton
Liu Q, Shinkle AA, Li Y, Monroe CW, Thompson LT, Sleightholme AE (2010) Non-aqueous chromium acetylacetonate electrolyte for redox flow batteries. Electrochem Commun 12(11):1634–1637
Newman J (1973) The fundamental principles of current distribution and mass transport in electrochemical cells. In: Dekker M (ed) Electroanalytical Chemistry, vol 6. p 187
Okada T, Møller-Holst S, Gorseth O, Kjelstrup S (1998) Transport and equilibrium properties of Nafion membranes with \(\text{ H }^{+}\) and \(\text{ Na }^{+}\) ions. J Electroanal Chem 442(1–2):137–145
Ruff I, Friedrich VJ, Csillag K (1972) Transfer diffusion. III. Kinetics and mechanism of the triiodide-iodide exchange reaction. J Phys Chem 76:162–165
Skyllas-Kazacos M (2003) Novel vanadium chloride/polyhalide redox flow battery. J Power Sources 124(1):299–302
Skyllas-Kazacos M, Kazacos G, Poon G, Verseema H (2010) Recent advances with UNSW vanadium-based redox flow batteries. Int J Energy Res 34(2):182–189
Skyllas-Kazacos M (2008) Vanadium/polyhalide redox flow battery. Google Patents
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biotechnol 26(4):417–426
Vafiadis H, Skyllas-Kazacos M (2006) Evaluation of membranes for the novel vanadium bromine redox flow cell. J Membr Sci 279(1–2):394–402
Wang JH, Kennedy JW (1950) Self-diffusion coefficients of sodium ion and iodide ion in aqueous sodium iodide solutions. J Am Chem Soc 72:2080–2083
Wang W, Luo Q, Li B, Wei X, Li L, Yang Z (2013) Recent progress in redox flow battery research and development. Adv Funct Mater 23(8):970–986
Wen YH, Cheng J, Ma PH, Yang YS (2008) Bifunctional redox flow battery-1 V(III)/V(II)-glyoxal(\(\text{ O }_{2}\)) system. Electrochim Acta 53(9):3514–3522
Zhang W, Stone HA, Sherwood JD (1996) Mass transfer at a microelectrode in channel flow. J Phys Chem 100(22):9462–9464
Acknowledgements
We thank the financial supports provided by Solvay Aubervilliers for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parant, H., Muller, G., Le Mercier, T. et al. Complete study of a millifluidic flow battery using iodide and ferricyanide ions: modeling, effect of the flow and kinetics. Microfluid Nanofluid 21, 171 (2017). https://doi.org/10.1007/s10404-017-2009-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-2009-1