Abstract
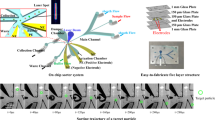
A low-cost, microfluidic fluorescence-activated cell sorting (μFACS) microchip integrated with two piezoelectric lead–zirconate–titanate actuators was demonstrated for automated, high-performance mammalian cell analysis and enrichment. In this PDMS–glass device, cells were hydrodynamically focused into a single file line in the lateral direction by two sheath flows, and then interrogated with a forward scattering and confocal fluorescent detection system. The selected cells were displaced transversely into a collection channel by two piezoelectric actuators that worked in a pull–push relay manner with a minimal switching time of ~0.8 ms. High detection throughput (~2500 cells/s), high sorting rate (~1250 cells/s), and high sorting efficiency (~98%) were successfully achieved on the μFACS system. Six cell mixture samples containing 22.87% of GFP-expressing HeLa cells were consecutively analyzed and sorted on the chip, revealing a stable sorting efficiency of 97.7 ± 0.93%. In addition, cell mixtures containing 37.65 and 3.36% GFP HeLa cells were effectively enriched up to 83.82 and 78.51%, respectively, on the microchip, and an enrichment factor of 105 for the low-purity (3.36%) sample was successfully obtained. This fully enclosed, disposable microfluidic chip provides an automated platform for low-cost fluorescence-based cell detection and enrichment, and is attractive to applications where cross-contamination between runs and aerosol hazard are the primary concerns.






Similar content being viewed by others
References
Amini H, Lee W, Di Carlo D (2014) Inertial microfluidic physics. Lab Chip 14:2739–2761
Cao Z et al (2013) Droplet sorting based on the number of encapsulated particles using a solenoid valve. Lab Chip 13:171–178
Chen CH, Cho SH, Chiang HI, Tsai F, Zhang K, Lo YH (2011) Specific sorting of single bacterial cells with microfabricated fluorescence-activated cell sorting and tyramide signal amplification fluorescence in situ hybridization. Anal Chem 83:7269–7275
Chen Y, Wu TH, Kung YC, Teitell MA, Chiou PY (2013) 3D pulsed laser-triggered high-speed microfluidic fluorescence-activated cell sorter. Analyst 138:7308–7315
Chen Y, Chung AJ, Wu TH, Teitell MA, Di Carlo D, Chiou PY (2014) Pulsed laser activated cell sorting with three dimensional sheathless inertial focusing. Small 10:1746–1751
Cho SH, Chen CH, Tsai FS, Godin JM, Lo YH (2010) Human mammalian cell sorting using a highly integrated micro-fabricated fluorescence-activated cell sorter (microFACS). Lab Chip 10:1567–1573
Duffy DC, McDonald JC, Schueller OJ, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984
Franke T, Braunmuller S, Schmid L, Wixforth A, Weitz DA (2010) Surface acoustic wave actuated cell sorting (SAWACS). Lab Chip 10:789–794
Fu AY, Spence C, Scherer A, Arnold FH, Quake SR (1999) A microfabricated fluorescence-activated cell sorter. Nat Biotechnol 17:1109–1111
Fu AY, Chou HP, Spence C, Arnold FH, Quake SR (2002) An integrated microfabricated cell sorter. Anal Chem 74:2451–2457
Gawad C, Koh W, Quake SR (2016) Single-cell genome sequencing: current state of the science. Nat Rev Genet 17:175–188
Godin J, Chen C-H, Cho SH, Qiao W, Tsai F, Lo Y-H (2008) Microfluidics and photonics for bio-system-on-a-chip: a review of advancements in technology towards a microfluidic flow cytometry chip. J Biophotonics 1:355–376
Gray DW, Gohde W, Carter N, Heiden T, Morris PJ (1989) Separation of pancreatic islets by fluorescence-activated sorting. Diabetes 38(Suppl 1):133–135
Ho CT, Lin RZ, Chang HY, Liu CH (2005) Micromachined electrochemical T-switches for cell sorting applications. Lab Chip 5:1248–1258
Holmes KL et al (2014) International society for the advancement of cytometry cell sorter biosafety standards. Cytom A 85:434–453
Ibrahim SF, van den Engh G (2003) High-speed cell sorting: fundamentals and recent advances. Curr Opin Biotechnol 14:5–12
Jaye DL, Bray RA, Gebel HM, Harris WA, Waller EK (2012) Translational applications of flow cytometry in clinical practice. J Immunol 188:4715–4719
Kim HJ, Moon HS, Kwak BS, Jung HI (2011) Microfluidic device to separate micro-beads with various fluorescence intensities. Sens Actuators B Chem 160:1536–1543
MacDonald MP, Spalding GC, Dholakia K (2003) Microfluidic sorting in an optical lattice. Nature 426:421–424
Macey MG (ed) (2007) Flow cytometry: principles and applications. Humana Press Inc., Totowa
Magbanua MJ, Park JW (2013) Isolation of circulating tumor cells by immunomagnetic enrichment and fluorescence-activated cell sorting (IE/FACS) for molecular profiling. Methods 64:114–118
Mao X, Lin SC, Dong C, Huang TJ (2009) Single-layer planar on-chip flow cytometer using microfluidic drifting based three-dimensional (3D) hydrodynamic focusing. Lab Chip 9:1583–1589
Naujok O, Kaldrack J, Taivankhuu T, Jorns A, Lenzen S (2010) Selective removal of undifferentiated embryonic stem cells from differentiation cultures through HSV1 thymidine kinase and ganciclovir treatment. Stem Cell Rev 6:450–461
Osborne GW (2011) Recent advances in flow cytometric cell sorting. Methods Cell Biol 102:533–556
Perroud TD, Kaiser JN, Sy JC, Lane TW, Branda CS, Singh AK, Patel KD (2008) Microfluidic-based cell sorting of Francisella tularensis infected macrophages using optical forces. Anal Chem 80:6365–6372
Preffer F, Dombkowski D (2009) Advances in complex multiparameter flow cytometry technology: applications in stem cell research. Cytom B Clin Cytom 76:295–314
Schmid I, Lambert C, Ambrozak D, Perfetto SP (2007) Standard safety practices for sorting of unfixed cells. Curr Protoc Cytom 3.6.1–3.6.20. doi:10.1002/0471142956.cy0306s39
Sciambi A, Abate AR (2015) Accurate microfluidic sorting of droplets at 30 kHz. Lab Chip 15:47–51
Shapiro HM (ed) (2005) Practical flow cytometry, 4th edn. Wiley Online Library, New York
Shirasaki Y, Tanaka J, Makazu H, Tashiro K, Shoji S, Tsukita S, Funatsu T (2006) On-chip cell sorting system using laser-induced heating of a thermoreversible gelation polymer to control flow. Anal Chem 78:695–701
Sugino H, Ozaki K, Shirasaki Y, Arakawa T, Shoji S, Funatsu T (2009) On-chip microfluidic sorting with fluorescence spectrum detection and multiway separation. Lab Chip 9:1254–1260
Wang MM et al (2005) Microfluidic sorting of mammalian cells by optical force switching. Nat Biotechnol 23:83–87
Wolff A et al (2003) Integrating advanced functionality in a microfabricated high-throughput fluorescent-activated cell sorter. Lab Chip 3:22–27
Wu L, Chen P, Dong Y, Feng X, Liu BF (2013) Encapsulation of single cells on a microfluidic device integrating droplet generation with fluorescence-activated droplet sorting. Biomed Microdevices 15:553–560
Wyatt Shields C IV, Reyes CD, Lopez GP (2015) Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 15:1230–1249
Yu ZT, Aw Yong KM, Fu J (2014) Microfluidic blood cell sorting: now and beyond. Small 10:1687–1703
Zhuang B et al (2016) A fully integrated and automated microsystem for rapid pharmacogenetic typing of multiple warfarin-related single-nucleotide polymorphisms. Lab Chip 16:86–95
Acknowledgements
We thank Dong Wang and Lei Wang at the National Engineering Research Center for Beijing Biochip Technology for their valuable advices on system development. We also thank the Cell Facility in the Tsinghua Center of Biomedical Analysis for the assistance on the BD FACSCalibur™ instrument. Microchip fabrication was conducted at the Microfabrication Laboratory, the National Engineering Research Center for Beijing Biochip Technology, China. This work was supported by the National Natural Science Foundation of China (No. 81341081).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (Video S1, AVI 10083 kb)
Rights and permissions
About this article
Cite this article
Cheng, Z., Wu, X., Cheng, J. et al. Microfluidic fluorescence-activated cell sorting (μFACS) chip with integrated piezoelectric actuators for low-cost mammalian cell enrichment. Microfluid Nanofluid 21, 9 (2017). https://doi.org/10.1007/s10404-017-1847-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-1847-1