Abstract
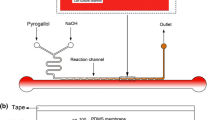
Oxygen participates in numbers of cellular activities and behaviors in both normal and pathological tissues. In physiological microenvironment, oxygen tension is generally below 21 % and varies in different species, states and regions of organs. However, present studies of cellular behavior in vitro are performed in an ambient level, which is not conformity to the reality in vivo. In this study, a microfluidic device was developed to generate controllable oxygen tensions on a multiple-channel array chip for high-throughput drug screening. Controlling various concentrations of chemical reagents with confined flow rate, specific oxygen tensions can be established from 1.6 to 21 %, where the oxygen tension of each channel can be modulated in demand. When the concentrations of pyrogallol change from 100 to 700 μg/mL with the flow rate of 5 μL/min, oxygen tensions in cell chambers range from 12.5 to 3.87 %. Pyrogallol with the concentration of 0 μg/mL is used as the control group to obtain 20.9 % oxygen condition. The developed microfluidic chip was used to investigate the cytotoxicity of TPZ and cisplatin, and the results demonstrate different manners of two oxygen-sensitive anti-tumor drugs in oxygen-dependent cytotoxic responses. Due to its character, the microfluidic device is believed to establish any desired and measurable oxygen tension distribution for pharmacology development, which is promising to improve efficiency and reduce tedious operation for pharmaceutical studies.






Similar content being viewed by others
References
Adams GE (1981) Hypoxia-mediated drugs for radiation and chemotherapy. Cancer 48:696–707
Brennan MD, Rexius-Hall ML, Elgass LJ, Eddington DT (2014) Oxygen control with microfluidics. Lab Chip 14:4305–4318
Brown JM (1990) Tumor hypoxia, drug resistance, and metastases. J Natl Cancer Inst 82:338–339
Brown JM (1993) SR 4233(Tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer 67:1163–1170
Brown JM, Wilson WR (2004) Exploiting tumor hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
Brown KE, Yamada KM (1995) The role of integrins during vertebrate development. Semin Dev Biol 6:69–77
Byrne MB, Leslie MT, Gaskins HR, Kenis Paul JA (2014) Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol 32:556–563
Chen H, Yuan L, Song W, Wu Z, Li D (2008) Biocompatible polymer materials: role of protein-surface interactions. Prog Polym Sci 33:1059–1087
Chen YA, King AD, Shih H, Peng CC, Wu CY, Liao WH, Tung YC (2011) Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip 11:3626–3634
Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, Kim HJ, Jeong H, Kim HM, Hwang D, Kim HS, Choi S (2015) Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One 10:1–21
Cole MA, Voelcker NH, Thissen H, Griesser HJ (2009) Stimuli-responsive interfaces and systems for the control of protein surface and cell surface interactions. Biomaterials 30:1827–1850
Darribere T, Koteliansky VE, Chernousov MA, Akiyama SK, Yamada KM, Thiery JP, Boucaut JC (1992) Distinct regions of human fibronectin are essential for fibril assembly in an in vivo developing system. Dev Dyn 194:63–70
Delahoussaye YM, Wouters BG, Evans JE (1997) Intranuclear metabolism of tirapazamine by matrix-associated reductases. Proc Am Assoc Cancer Res 38:163
Dorie MJ, Brown JM (1993) Tumor-specific schedule-dependent interaction between tirapazamine and cisplatin. Cancer Res 53:4633–4636
Elwell JH, Siim BG, Evuns JW, Brown JM (1997) Adaptation of human tumor cells to tirapazamine under aerobic conditions. Biochem Pharmacol 54:249–257
Fieser LF (1924) A new absorbent for oxygen in gas analysis. J Am Chem Soc 46:2639–2647
Gomez-Sjoberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR (2007) Versatile, fully automated, microfluidic cell culture system. Anal Chem 79:8557–8563
Hung SP, Ho JH, Shih Yu-RuV, Ting Lo, Lee OK (2012) Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res 30:260–266
Kallman RF, Dorie MJ (1986) Tumor oxygenation and reoxygenation during radiation therapy: their importance in predicting tumor response. Int J Radiat Oncol Biol Phys 12:681–685
Kerbel RS (2008) Molecular origins of cancer: tumor angiogenesis. N Engl J Med 358:2039–2042
Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M (2003) Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 94:1021–1028
Kovacs MS, Hocking DJ, Evans JW, Siim BG, Wounters BG, Brown JM (1999) Cisplatin anti-tumour potentiation by tirapazamine results from hypoxia-dependent cellular sensitization to cisplatin. Br J Cancer 80:1245–1251
Lo JF, Sinkala E, Eddington DT (2010) Oxygen gradients for open well cellular cultures via microfluidic substrate. Lab Chip 10:2394–2401
Miyamoto S, KatzBZ Lafrenie RM, Yamada KM (1998) Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Annal NY Acad Sci 857:119–129
Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS (2001) Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res 2:217–223
Pennacchietti S, Michieli P, Galluzzo M (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3:347–361
Polinkovsky M, Gutierrez E, Levchenko A, Groisman A (2009) Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab Chip 9:1073–1084
Rexius-Hall ML, Mauleon G, Malik AB, Jalees Rehman, Eddington DT (2014) Microfluidic platform generates oxygen landscapes for localized hypoxic activation. Lab Chip 14:4688–4695
Roach P, Farrar D, Perry CC (2005) Interpretation of protein adsorption: surface induced conformational changes. J Am Chem Soc 127:8168–8173
Rofstad EK (2000) Microenvironment-induced cancer metastasis. Int J Radiat Biol 76:589–605
Shiku H, Saito T, Wu CC, Yasukawa T, Yokoo M, Abe H, Matsue T, Yamada H (2006) Oxygen permeability of surface-modified poly(dimethylsiloxane) characterized by scanning electrochemical microscopy. Chem Lett 35:234–235
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279
Skolimowski M, Nielsen MW, Emneus J, Molin S, Taboryski R, Sternberg C, Dufva M, Geschke O (2010) Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip 10:2162–2169
Subarsky P, Hill RP (2003) The hypoxic tumor microenvironment and metastatic progression. Clin Exp Metastasis 20:237–250
Teicher BA (1994) Hypoxia and drug resistance. Cancer Metastasis Rev 13:139–168
Wang JB, Zhou Y, Qiu HW, Huang H, Sun CH, Xi JZ, Huang YY (2009) A chip-to-chip nanoliter microfluidic dispenser. Lab Chip 9:1831–1835
Wang ZH, Liu ZX, Lili Li, Liang QL (2015) Investigation into the hypoxia dependent cytotoxicity of anticancer drugs under oxygen gradient in a microfluidic device. Microfluid Nanofluid 19:1271–1279
Acknowledgments
We greatly appreciate the financial support from the Ministry of Science and Technology (2013ZX09507005), National Natural Science Foundation of China (21175080, U1333132, 20121312013) and Beijing Municipality (Z131100006513009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Li, L., Liu, Z. et al. A microfluidic chip of multiple-channel array with various oxygen tensions for drug screening. Microfluid Nanofluid 20, 97 (2016). https://doi.org/10.1007/s10404-016-1762-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-016-1762-x