Abstract
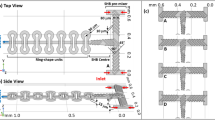
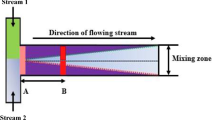
The rapid mixing of fluids passing through a microfluidic channel is very important for various applications of microfluidic systems. It has been a great challenge to achieve highly efficient mixing in a microfluidic system because it is very difficult to generate turbulence in a submillimeter-size channel at low Reynolds numbers (Re). In this paper, we fabricated a pillar obstruction microfluidic mixer and evaluated its mixing efficiency at various flow rates. The mixing behavior of confluent streams was estimated using a fluorescence microscope. Three different sets of miscible solutions (phosphate-buffered solution, gold nanocolloids and 20% glycerol), with Rhodamine 6G aqueous solution, were used as sample laminar flows. According to our experimental results, the pillar obstruction microfluidic mixer shows an excellent mixing performance in the low Re range. Here, the mixing performance was strongly dependent on the characteristic viscosity changes of different sets of miscible solutions. The pillar obstruction microfluidic mixer designed here is expected to benefit a wide range of lab-on-a-chip applications because fabrication is very simple and the mixing efficiency is excellent at low Re.




Similar content being viewed by others
References
Bessoth FG, de Mello AJ, Manz A (1999) Microstructure for efficient continuous flow mixing. Anal Commun 36:213–215
Bhagat AAS, Peterson ETK, Papautsky I (2007) A passive planar micromixer with obstructions for mixing at low Reynolds numbers. J Micromech Microeng 17:1017–1024
Chen L, Choo J (2008) Recent advances on surface-enhanced Raman scattering detection technology for microfluidic chips. Electrophoresis 29:1815–1828
Chen L, Lee S, Choo J, Lee EK (2008a) Continuous dynamic flow micropumps for microfluid manipulation. J Micromech Microeng 18:013001
Chen L, Lee S, Lee M, Lim C, Choo J, Park JY, Lee S, Joo SW, Lee KH, Choi YW (2008b) DNA hybridization detection in a microfluidic channel using two fluorescently labeled nucleic acid probes. Biosens Bioelectron 23:1878–1882
De Mello AJ (2006) Control and detection of chemical reactions in microfluidic systems. Nature 442:394–402
Den Toonder J, Bos F, Broer D, Filippini L, Gillies M, de Goede J, Mol T, Reijme M, Talen W, Wilderbeek H, Khatavkar V, Anderson P (2008) Artificial cilia for active micro-fluidic mixing. Lab Chip 8:533–541
El Moctar AO, Aubry N, Batton J (2003) Electro-hydrodynamic micro-fluidic mixer. Lab Chip 3:273–280
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodispersed gold suspensions. Nat Phys Sci 241:20–22
He B, Burke BJ, Zhang X, Zhang R, Regnier FE (2001) A picoliter-volume mixer for microfluidic analytical systems. Anal Chem 73:1942–1947
Jung J, Chen L, Lee S, Kim S, Seong GH, Choo J, Lee EK, Oh CH, Lee S (2007) Fast and sensitive DNA analysis using changes in the FRET signals of molecular beacons in a PDMS microfluidic channel. Anal Bioanal Chem 387:2609–2615
Leung SA, Winkle RF, Wootton RCR, de Mello AJ (2005) A method for rapid reaction optimisation in continuous-flow microfluidic reactors using online Raman spectroscopic detection. Analyst 130:46–51
Liu AL, He FY, Wang K, Zhou T, Lu Y, Xia XH (2005) Rapid method for design and fabrication of passive micromixers in microfluidic devices using a direct-printing process. Lab Chip 5:974–978
Liu RH, Stremler MA, Sharp KV, Olsen MG, Santiago JG, Adrian RJ, Aref H, Beebe DJ (2000) Passive mixing in a three-dimensional serpentine microchannel. J Microelectromech Syst 9:190–197
Lu LH, Ryu KS, Lu C (2002) A magnetic microstirrer and array for microfluidic mixing. J Microelectromech Syst 11:462–469
Mengeaud V, Josserand J, Girault HH (2002) Mixing processes in a zigzag microchannel: finite element simulations and optical study. Anal Chem 74:4279–4286
Nguyen NT, Wu Z (2005) Micromixers—a review. J Micromech Microeng 15:R1–R16
Park T, Lee M, Choo J, Kim YS, Lee EK, Kim DJ, Lee SH (2004) Analysis of passive mixing behavior in a PDMS microfluidic channel using confocal fluorescence and Raman microscopy. Appl Spectrosc 58:1172–1179
Sasaki N, Kitamori T, Kim HB (2006) AC electroosmotic micromixer for chemical processing in a microchannel. Lab Chip 6:550–554
Slentz BE, Penner NA, Regnier FE (2002) Capillary electrochromatography of peptides on microfabricated poly(dimethylsiloxane) chips modified by cerium(IV)-catalyzed polymerization. J Chromatogr A 948:225–233
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem Int Ed 42:767–772
Sritharan K, Strobl CJ, Schneider MF, Wixforth A, Guttenberg Z (2006) Acoustic mixing at low Reynolds numbers. Appl Phys Lett 88:054102
Stroock AD, Dertinger SKW, Ajdari A, Mezic I, Stone HA, Whitesides GM (2002) Chaotic mixer for microchannels. Science 295:647–651
Unger KK, Skudas R, Schulte MM (2008) Particle packed columns and monolithic columns in high-performance liquid chromatography—comparison and critical appraisal. J Chromatogr A 1184:393–415
Wang H, Iovenitti P, Harvey E, Masood S (2002) Optimizing layout of obstacles for enhanced mixing in microchannels. Smart Mater Struct 11:662–667
Yang J, Pi XT, Zhang LG, Liu XS, Yang J, Cao Y, Zhang WX, Zheng XL (2007) Diffusion characteristics of a T-type microchannel with different configurations and inlet angles. Anal Sci 23:697–703
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (Grant numbers R01-2007-000-20238-0 and R11-2008-044-01002-0), the National Cancer Center of Korea (Grant number 0620400-1) and the Seoul Research and Business Development Program (Grant number 10574). J.C. also thanks for the financial support from the Korea Research Foundation (Grant number C00170).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Wang, G., Lim, C. et al. Evaluation of passive mixing behaviors in a pillar obstruction poly(dimethylsiloxane) microfluidic mixer using fluorescence microscopy. Microfluid Nanofluid 7, 267–273 (2009). https://doi.org/10.1007/s10404-008-0386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-008-0386-1