Abstract
This was a single-site cohort study to evaluate the safety of a new transcervical device (VizAblate™) combining real-time intrauterine sonography with radiofrequency (RF) ablation for the treatment of fibroids. Nineteen women with uterine fibroids received treatment with the VizAblate System in a closed abdomen setting prior to hysterectomy. Twelve of these subjects underwent an immediate abdominal hysterectomy after radiofrequency ablation (acute group), while the remaining seven underwent hysterectomy on post-ablation days 16 and 17 (subacute group). Uteri were sectioned and stained with the viability stain triphenyltetrazolium chloride (TTC) to quantify fibroid ablation dimensions and assess the serosa for thermal injury. Subjects in the subacute group were treated with the VizAblate System under conscious sedation; they provided pain and tolerability data for the interval from ablation through hysterectomy, and indicated overall procedural satisfaction. Twenty-two ablations ranging from 1.8 to 36.2 cm3 were created among 19 subjects within 20 fibroids and one region of adenomyosis. There were no complications or thermal serosal injury. For subjects in the subacute group receiving one ablation, the mean total procedure time was 25.8 ± 6.0 min (range 18–32 min). All subjects in the subacute group were discharged within 2 h of the VizAblate procedure. For fibroids ≤ 5 cm, 67.2% ± 27.0% of the fibroid volume was ablated (range 15–100%; median 75%). Transcervical RF ablation of fibroids under intrauterine sonographic guidance with the VizAblate system can be accomplished with a high degree of reliability and without adverse events.
Similar content being viewed by others
Introduction
Uterine fibroids are the most prevalent benign uterine tumors and have an age-specific cumulative incidence in the USA that is nearly 70% among white women and greater than 80% among black women [1]. Uterine fibroids are estimated to occur in approximately 20–25% of adult women overall with symptoms that may involve menorrhagia and subfertility as well as bulk symptoms. Despite numerous alternatives to hysterectomy, over 200,000 hysterectomies are performed for fibroids annually in the USA [1, 2]. There is also a lack of consensus regarding optimal therapy for uterine fibroids, so that more than 150 years after the first abdominal hysterectomy for fibroids, there is no definitive clinical evidence for what constitutes the “best” treatment for fibroids [3].
Radiofrequency ablation (RFA) has been used as a fibroid treatment modality since the early 1990s, with multiple clinical studies confirming its safety and efficacy [4–9]. It has been shown that radiofrequency ablation results in heat/thermal fixation and coagulative necrosis within the treated fibroids [9, 10]. Recent studies have been performed using RFA in conjunction with simultaneous, real-time sonography to enable volumetric ablations that result in volume reduction and symptomatic relief [8, 9, 11].
Cho and colleagues performed transcervical fibroid RFA in 153 women who were followed up to 18 months post-treatment [8]. Symptomatic relief was assessed through the Uterine Fibroid Symptom-Quality of Life questionnaire; the authors reported a 91% reduction in symptoms and a 46% improvement in quality of life. They demonstrated a 73% mean reduction in fibroid volume with only 4.3% (6/153) of the women requiring reintervention. Ghezzi and colleagues performed percutaneous RFA on up to three fibroids per patient in 25 women and followed them for 12 to 36 months. These patients all demonstrated symptomatic improvement and showed a mean 59% improvement in their quality of life with a 4% (1/25) reintervention rate at 1 year and as much as an 84% reduction in mean fibroid volumes at 36 months [9].
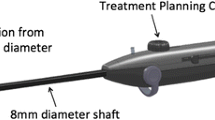
The VizAblate System™ (Gynesonics; Redwood City, CA) is a novel transcervical device that combines radiofrequency ablation for treatment with intrauterine sonography for lesion targeting. The VizAblate System (Fig. 1) consists of an intrauterine ultrasound (IUUS) probe and a single-use, disposable articulating handpiece that are combined into a single treatment device (Fig. 2). Other components of the VizAblate System include a custom RF generator and an ultrasound system with a custom graphical user interface (GUI). These uniquely integrated components provide the gynecologist with a high-quality image-guided treatment system. The VizAblate IUUS probe is used to localize fibroids from within the uterine cavity and guide placement of the VizAblate needle electrodes into targeted fibroids. Once positioning has been confirmed, therapeutic RF energy is delivered to the fibroid according to a fixed treatment cycle that is dependent on the desired ablation size.
The VizAblate GUI incorporates a treatment overlay (“treatment guides”), consisting of several graphical elements intended to assist the physician with the proper introducer and electrode placement and achieve safe ablation of the targeted fibroid. These graphical elements include the ablation zone (a red oval), the thermal safety border (a green oval), and additional elements for alignment of the overlay with the actual introducer and electrode positions as visualized in the ultrasound image (Fig. 3). The Ablation Zone represents the dimensions of an average ablation generated for the selected electrode deployment size. The thermal safety border represents the distance from the needle electrode beyond which tissue is safe from potential thermal damage; this is important from a safety perspective as there will be thermal heating of tissue beyond the area of the ablation that is represented by the ablation zone. The ablation zone and thermal safety border overlays have been validated through bench investigations of bovine muscle, direct uterine serosal temperature measurements using thermocouples and infrared thermography, and subsequent pathological analysis. The VizAblate GUI guides the physician through the prescribed procedure workflow, requiring specific steps in order to initiate RF treatment. This enables the gynecologist to select the appropriate needle electrode deployment width that maximizes ablation volume while avoiding thermal damage to the uterine serosal surface.
IUUS image of a submucosal fibroid with the VizAblate treatment guides (ablation zone in red, thermal safety border in green) indicated. The mean region treatment delineates where the ablation will occur and the thermal safety border demarcates the extent of thermal spread beyond the ablation. The serosa is visible as an echogenic border around the uterus (yellow arrow)
The shape of the ablations created by the VizAblate System are ellipsoidal, with an approximate maximum length of 5.0 cm and width of 4.0 cm. Single ablations with the current VizAblate System can ablate fibroids up to 5 cm in diameter. The ablation dimensions depend on how deeply the needle electrodes are deployed as well as the treatment time at temperature (105°C). The ablation width is adjustable in half-centimeter increments between 1.0 and 4.0 cm. The system automatically modulates RF power to maintain the target temperature for a prescribed duration, depending on the size of the desired ablation. The user simply matches the size of the Ablation Zone to that of the targeted fibroid, and ensures that the Thermal Safety Border is within the serosal margin at all times; the software algorithm then selects the duration of treatment by design based on the size of the Ablation Zone. Four minutes at temperature is used to ablate fibroids from 1 to 2 cm in diameter, 6 min is necessary to ablate fibroids greater than 2 cm up to 3.5 cm in diameter, and 9 min is required to ablate fibroids 3.5 cm or larger. These capabilities allow the physician to safely tailor the ablation position and volume to the particular fibroid and its location relative to the serosa. In many cases, a fibroid can be completely ablated when situated sufficiently distant from the serosa. When a fibroid is too close to the serosa, the physician can still use the treatment guides to safely ablate a significant portion of the fibroid's volume while still maintaining safety with respect to the serosa.
The VizAblate System has been developed in an open abdomen, perihysterectomy setting involving 89 treated subjects. The use of concurrent laparotomy in that work enabled serosal temperature monitoring and also permitted the packing of bowel out of harm's way. In actual use, the VizAblate System would be introduced into the endometrial cavity via a transcervical approach in order to ablate submucosal, intramural, and transmural fibroids without incisions and without a requirement for general anesthesia.
This report describes the initial safety, tolerability, and procedure times of the VizAblate System when used under conditions for its anticipated use, in a closed abdomen setting, in women with uterine fibroids.
Materials and methods
Patient selection
This study was a prospective, non-randomized, single-arm, single-site trial (Canadian Task Force classification II-2) involving subjects with uterine fibroids who had elected to undergo hysterectomy for menorrhagia and other uterine symptomatology. The initial protocol and subsequent revisions were approved by the Ethics Committee of the Universidad Autónoma de Nuevo León Facultad de Medicina in Monterrey, Mexico. The procedures followed were in accordance with the ethical standards of the national responsible committee on human experimentation (Comisión Federal para la Protección contra Riesgos Sanitarios/Federal Commission for the Protection against Sanitary Risk; COFEPRIS) and with the Helsinki Declaration of 1975, as revised in 2000. All enrolled subjects provided written informed consent for treatment with the VizAblate System prior to hysterectomy.
Each subject underwent screening that included transvaginal sonography, endometrial biopsy, and a pregnancy test. Women were eligible for inclusion if they were 25 years of age or older, had previously agreed to undergo elective hysterectomy, and had between one and five fibroids ranging from 1 to 5 cm in diameter on a screening sonographic examination.
Procedure
There were two non-randomized subject cohorts: the acute group (subjects received hysterectomy immediately after closed abdomen use of the VizAblate System) and the subacute group (subjects underwent delayed hysterectomy on postablation days 16 and 17). On the procedure date, subjects in the acute group underwent epidural catheter placement for regional anesthesia as they were to undergo total abdominal hysterectomy immediately following transcervical radiofrequency ablation. The women in the subacute group who were to undergo a delayed hysterectomy received conscious sedation (intravenous midazolam and fentanyl) with paracervical blockade (lidocaine) at the time of transcervical radiofrequency ablation. For both groups, a dispersive electrode pad was placed bilaterally on the anterior thighs with a thermocouple positioned at each leading edge for skin temperature monitoring. Intraoperative evaluation included transvaginal sonography to confirm the presence, location, and fibroid sizes present within the uterus.
After achieving cervical dilatation to 8 mm, the VizAblate articulating handpiece containing the IUUS probe was inserted transcervically into the subject's uterus. Several milliliters (generally 10–15 ml) of hypotonic fluid in the form of 1.5% glycine were infused through the device for acoustic coupling. Leiomyomata were then visualized and mapped in a systematic fashion within the uterus.
Throughout this study, the investigator was asked to treat fibroids in a manner consistent with his expected “real world” clinical use. That is, to ablate those fibroids having a reasonable probability of being symptomatic, while ensuring subject safety and attempting to ablate at least 50% of each fibroid's volume. In doing so, the investigator determined the best ablation size and location for each individual fibroid, and whether one or more ablations should be performed.
The treated fibroids each received from one to two volumetric ablations, ranging from 1 to 4 cm in width (in half-centimeter increments) using radiofrequency energy (maximum output 150 W) for 4–9 min at steady temperature of 105°C. As indicated, ablation dimensions are a function of both the needle electrode deployment width and treatment time.
All subjects were treated with the VizAblate System by a single surgeon. After treatment with VizAblate, the women underwent total abdominal hysterectomy either immediately (acute group) or just over 2 weeks later (subacute group). Following immediate or delayed hysterectomy, uteri were sectioned and stained for viability with TTC (Fig. 4) to quantify the fibroid ablation dimensions and the percentage of the fibroid volume that was ablated.
TTC-stained hysterectomy specimen from a subject in the subacute group with two ablated intramural fibroids in the same uterus. The pink tissue represents viable, non-ablated tissue, whereas the white region indicates the ablation. The fibroid on the left is a 2.8 × 2.5 × 2.1-cm intramural leiomyoma in which 100% of the fibroid volume has been ablated. The fibroid on the right is a 4.1 × 3.7 × 3.8-cm intramural fibroid; 90% of the fibroid's volume was ablated. There is black ink on the anterior surface applied by the pathologist, seen here below the peritoneal reflection, indicating the anterior surface of the uterus to maintain the orientation during subsequent pathological analysis
Procedure times were recorded for all subjects in the subacute group based on real-time videos of each procedure; these times were taken from the start of transvaginal sonography to the end of RF ablation with the VizAblate System. Procedure times for women in the acute group were not considered because of confounding ancillary measurements, image optimization procedures as part of the device development process at that time, and additional delays owing to the need for subsequent laparotomy in these subjects. Subjects in the subacute group were interviewed regarding pain during the VizAblate procedure and recovery using a 10-point numeric variable analog scale (VAS) before discharge from the hospital after their VizAblate procedure and again when they returned for their hysterectomy; the latter interview covered the interval between the VizAblate procedure and the hysterectomy over 2 weeks later.
Findings
Acute group
A total of 13 subjects were enrolled and 12 were treated. One enrolled subject was not treated with the VizAblate system due to the presence of apparent adenomyosis upon sonography in the operating room, with uncertainty as to whether the visualized lesion represented a fibroid or an adenomyotic cyst. She underwent her scheduled hysterectomy where pathologic evaluation demonstrated adenomyosis and a small fibroid.
Of the 12 treated subjects, one was found on pathology examination to have had adenomyosis rather than a fibroid; the screening transvaginal ultrasound results were not suggestive of adenomyosis, even in retrospect. This subject was excluded from the ablation percentage results, as precise tissue boundaries for the region of adenomyosis were not obtainable. In all, a total of 13 individual lesions were treated, representing 12 fibroids and one region of adenomyosis. There were 14 ablations in total; one of the 12 fibroids received two ablations and one subject had two fibroids that each received single ablations.
The maximum treated fibroid dimensions, based on measurements from the hysterectomy specimens, ranged from 0.8 cm (0.3 cm3) to 5.1 cm (69.5 cm3) in diameter. All targeted fibroids contained volumetric ablations ranging from 1.9 cm (1.8 cm3) to 4.0 cm (30.9 cm3; dual overlapping ablations) in diameter, depending on the clinically selected ablation size. The needle electrode deployment sizes selected during the procedures ranged from 1 to 3.5 cm in width.
The mean percentage of ablated fibroid volume in the 11 treated fibroids ≤ 5.0 cm in diameter was 62.3% ± 27.6% (range 15–100%; median 60%). In five of these 11 fibroids (45.5%), at least 75% of the fibroid volume was ablated. The mean percentage ablation in all 12 treated fibroids, irrespective of fibroid size, was 62.1% ± 26.3% (range 15–100%; median 60%).
With regard to serosal safety, no thermal injury to the uterine serosa was associated with any of the 13 fibroid ablations, either upon gross inspection at the time of hysterectomy or after pathologic examination. The maximum dispersive electrode pad temperature was 37.6°C. There were no complications and all subjects were discharged in good condition after hysterectomy.
Subacute group
Thirteen subjects were enrolled in the subacute group and seven were treated. A total of eight fibroids each received a single ablation with the clinically selected treatments ranging from 1.5 to 4.0 cm in width using radiofrequency energy (maximum output 150 W) for up to 9 min at a constant temperature of 105°C.
One enrolled subject withdrew early in order to pursue pharmacologic treatment, while another did not show up on the day of the procedure. Four additional subjects were not treated due to pathology detected on imaging findings in the operating room that were not amenable to treatment; one subject had imaging that was suggestive for adenomyosis; two subjects had purely subserosal myomata that could not be safely treated in a closed abdomen setting; one had a fibroid whose diameter measured considerably above the upper limit of 5.0 cm for inclusion within the study.
The maximum diameter of treated fibroids ranged from 1.6 to 7.4 cm (hysterectomy specimen measurements). Six of the eight fibroids were less than 5.0 cm in maximum diameter. All eight targeted fibroids contained volumetric ablations ranging from 2.5 cm3 (1.7 cm diameter) to 36.2 cm3 (4.1 cm diameter), depending on the clinically desired ablation size.
For all six treated fibroids ≤ 5 cm in diameter, the mean percentage of the fibroid that was ablated was 76.3% ± 25.5% (range 40–100%; median 85%). In four of these six fibroids (66.7%), at least 75% of the fibroid volume was ablated. The mean percentage ablation in all eight treated fibroids, irrespective of fibroid size, was 62.9% ± 33.3% (range 20–100%; median 65%).
The mean procedure duration from the start of transvaginal sonography to the end of RF ablation was 25.8 min ± 6.0 min (range 18–32 min) for the six procedures involving single fibroid ablations. The procedure time was 39 min for the one subject who had two separate fibroids ablated; each ablation involved 6 min of steady-state ablation at 105°C.
As with the acute group, none of the eight fibroid ablations had any evidence of serosal injury at laparotomy or at the time of pathologic evaluation. There were no immediate complications and all subjects were ambulatory and discharged in good condition within 2 h of the VizAblate procedure. The maximum dispersive electrode pad temperature was 36.5°C. Between the VizAblate procedure and hysterectomy 16–17 days later, there were no adverse events reported other than anticipated common side effects (cramping, spotting, and discharge). Six of seven (85.7%) subjects experienced a total of 13 instances of minor side effects such as spotting (four subjects), continuous bleeding for 6–10 days post-treatment (two subjects), clear discharge in three subjects and cramping in four subjects.
Procedure-associated pain levels (10-point VAS scale) averaged 0.9 ± 1.1 (range 0–3) and were similar during immediate recovery and at discharge after the VizAblate procedure. Return to normal activity averaged 3.3 ± 3.2 days (range 0.3–8 days), and the mean pain level from the time of the procedure to hysterectomy was 3.0 ± 2.1 (range 0–5). Table 1 lists the results for the recovery questionnaire.
Pooled data
For the acute and subacute groups combined, a total of 26 subjects were enrolled. Twenty-two ablations were performed on 20 fibroids in 19 subjects.
Uterine weights ranged from 103 to 513 g. The treated fibroid diameters ranged from 0.8 to 7.4 cm and the ablation volume ranged from 1.8 to 36.2 ml, depending on desired ablation size
For the 17 treated fibroids ≤ 5 cm in diameter that were treated in the acute and subacute substudies, the average pooled percentage of ablated fibroid volume was 67.2% ± 27.0% (range 15–100%; median 75%). At least 75% of the fibroid volume was ablated in nine of these 17 fibroids (52.9%). The mean percentage of ablated fibroid volume in all 20 fibroids treated for both groups, regardless of size, was 62.4% ± 28.3% (range 15–100%; median 60%). Grouped as well as pooled ablation results may be found in Table 2.
Discussion
Since the 1990s, several new modalities to treat uterine fibroids have emerged, including uterine artery embolization and magnetic resonance-guided focused ultrasound (MRgFUS). Uterine artery embolization is effective treatment, with a 20–28.4% failure rate after 5 years [12, 13]. Nonetheless, it is not considered appropriate for women who desire future fecundity and has been associated with postembolization syndrome and premature ovarian failure [13–18]. MR-guided focused ultrasound, like radiofrequency ablation methods, ablates fibroids using energy to generate hyperthermic tissue temperatures. Based on the published MRgFUS and RFA literatures, hyperthermic ablation leads to tissue necrosis, reduction in fibroid volume, and symptomatic relief [7–10, 19, 20]. MRgFUS, however, is not widely available and requires up to 3 h per treatment.
Radiofrequency energy and sonography are familiar to most gynecologists. In the past, attempts to ablate fibroids with radiofrequency energy (“myolysis”) were limited by the lack of concurrent imaging that necessitated multiple transserosal passes via laparoscopy or laparotomy in an attempt to effectively coagulate the myoma.
The VizAblate System uses a transcervical approach, in which fibroid ablation takes place under real-time visualization as an intrauterine sonography probe is built into the device. This built-in imaging removes the need for the physician to coordinate more than one device. The graphical interface delineates the boundaries of both ablation and thermal spread, enabling the gynecologist to avoid thermal injury to the serosa with its potential for adhesiogenesis and injury to bowel or bladder. Ablation volume is a function of time at temperature (105°C) and electrode deployment, so the gynecologist can choose the electrode deployment (which corresponds to a treatment time of 4, 6, or 9 min) that optimizes the fibroid ablation volume while maintaining safety.
As indicated, a single ablation with the VizAblate System can treat a fibroid up to 5 cm in diameter, although multiple ablations may be used to manage larger fibroids. For the 17 fibroids in this study that were no more than 5 cm in diameter, an average of 67.2% ± 27.0% of a targeted fibroid's volume was ablated (range 15–100%; median 75%). There was a large range in ablation percentage, but in most cases, a significant portion (up to 100%) of a targeted fibroid was successfully targeted and ablated. This percentage is under direct control of the gynecologist and depends on the placement of the treatment guides, and therefore the electrodes, within the target fibroid. Fibroids that lie closer to the endometrial cavity, namely submucous and many intramural fibroids, may be completely ablated through the use of the VizAblate System, depending on their size and location. Such fibroids are believed to be chiefly responsible for menorrhagia [21, 22]. For fibroids that lie close to the uterine serosa, the geometry may be such that the treating gynecologist will not necessarily ablate 100% of the fibroid's volume. This is dictated by the need to avoid thermal injury to the serosal surface, both to prevent adhesiogenesis as well as injury to adjacent viscera. The thermal safety border thus constrains the size and position of the fibroid ablation. However, it has been reported in the MRgFUS literature that it is not necessary to ablate 100% of a fibroid in order to provide fibroid volume reduction and durable symptomatic improvement [20, 23].
Previous to the work described in this report, the VizAblate System was safely used in 89 women who underwent fibroid ablation in an open abdomen setting. This permitted the bowel to be packed out of harm's way, and also enabled the recording of serosal temperatures during ablation through the use of thermocouples as well as an infrared camera. Unlike the previous work involving concurrent laparotomy, this current study examined the safety, tolerability, and procedure duration for the VizAblate System when used in a manner approaching real-world conditions in a closed abdomen setting. The initial cases (acute group) involved women who underwent hysterectomy immediately after fibroid ablation with the VizAblate System. This enabled assessment of the serosal surface, both intraoperatively and in the pathology laboratory, along with evaluation for any suspected thermal injury to adjacent bowel.
After demonstrating safety in the acute group, the VizAblate System was used in women who underwent a delayed hysterectomy (subacute group), 16–17 days post-ablation. These subjects also provided information relative to their recovery over the 2-week interval between the ablation and hysterectomy; such information would have been confounded by the performance of immediate hysterectomy in the acute group.
This study found no complications associated with closed abdomen use of the VizAblate System. In every subject, there was no evidence of thermal serosal injury at the time of hysterectomy or on pathologic examination of the extirpated uteri. No subject required readmission for any reason, nor did any subject need to return to the operating room. Not unexpectedly, some subjects experienced cramping, bleeding, and spotting over the 2 weeks following the procedure.
Pain scores in the subacute group following the procedure suggest that the VizAblate System may be successfully used with conscious sedation and paracervical blockade. Overall, the procedure was well tolerated, with minimal pain levels associated with fibroid ablation, and pain scores for the interval from the ablation procedure to hysterectomy averaging 3.0 ± 2.1 (range 0–5) out of a maximum of 10.
All treatments for uterine fibroids have their relative advantages. The VizAblate System does not involve uterine distension; only 10–15 ml of hypotonic fluid are required for acoustic coupling, similar to the volumes required for saline infusion sonography. Furthermore, there is no need to increase intrauterine pressures above mean arterial pressure. The combination of imaging and treatment in one device does not require the gynecologist to coordinate a separate ultrasound probe with the treatment device. In addition, intrauterine sonography may provide a higher resolution view of the uterus than that of standard transvaginal ultrasound. The gynecologist can also readily select ablation sizes to match those of the symptomatic fibroids, providing flexibility in creating volumetric ablations. The graphical user interface delineates the boundaries of the expected ablation and indicates the margins of thermal spread for the selected ablation size; this permits the gynecologist to constrain the ablation to the fibroid and avoid thermal damage to the serosa and adjacent structures. Use of the transcervical route avoids the need for laparoscopy and suggests this treatment modality could be suitable for an office setting. Procedure times, generally 30 min or less, are somewhat less than what has been reported on average for hysteroscopic myomectomy using a resectoscope (42.2 min; CI 39.7–44.7 min) [24].
There are some limitations to this study. Subjects were not screened for preexisting pelvic pain nor were many potential etiologies of menorrhagia (such as anovulation) excluded prior to enrollment, so the observed presence of post-procedure cramping and spotting in the subacute group may overestimate the true minor side effect incidence associated with transcervical radiofrequency ablation of fibroids. This study also did not assess the efficacy of treatment with the VizAblate System with regard to menorrhagia symptoms. A forthcoming, 48-subject trial will examine the efficacy of transcervical, intrauterine ultrasound-guided fibroid ablation with the VizAblate System and accrue additional safety and tolerability data.
Conclusions
The VizAblate System was used to safely target and ablate fibroids in 19 treated women who underwent a total of 22 radiofrequency ablations in a closed abdomen setting. VizAblate was well tolerated when used with conscious sedation and paracervical blockade, supporting its potential future use in an office setting. Procedure times were on the order of 30 min or less. The coupling of intrauterine sonography with radiofrequency ablation in a single device enables imaging, precise fibroid targeting, and treatment in one minimally invasive procedure.
References
Day Baird D, Dunson DB, Hill MC et al (2003) High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188(1):100–107
Dembek CJ, Pelletier EM, Isaacson KB et al (2007) Payer costs in patients undergoing uterine artery embolization, hysterectomy, or myomectomy for treatment of uterine fibroids. J Vasc Interv Radiol 18(10):1207–1213
Manyonda I, Sinthamoney E, Belli AM (2004) Controversies and challenges in the modern management of uterine fibroids. BJOG 111(2):95–102
Goldfarb HA (1992) Avoiding hysterectomy: Nd:YAG laser and bipolar coagulating needle. Clin Laser Mon 10(12):191–193
Goldfarb HA (1995) Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc 2(2):175–179
Bergamini V, Ghezzi F, Cromi A et al (2005) Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol 192(3):768–773
Carrafiello G, Recaldini C, Fontana F et al (2009) Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol 33(1):113–119. doi:10.1007/s00270-009-9707-3
Cho HH, Kim JH, Kim MR (2008) Transvaginal radiofrequency thermal ablation: a day-care approach to symptomatic uterine myomas. Aust N Z J Obstet Gynaecol 48(3):296–301
Ghezzi F, Cromi A, Bergamini V et al (2007) Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc 21(11):2081–2085
Luo X, Shen Y, Song W et al (2007) Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int J Gynecol Obstet 99(1):9–13
Iversen H, Lenz S (2008) Percutaneous ultrasound guided radiofrequency thermal ablation for uterine fibroids: a new gynecological approach. Ultrasound Obstet Gynecol 32(3):325
Kooij SM, Hehenkamp WJ, Volkers NA et al (2010) Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. Am J Obstet Gynecol 203(2):105.e1–105.e13
Spies JB, Bruno J, Czeyda-Pommersheim F et al (2005) Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol 106(5 Pt 1):933–939
Homer H, Saridogan E (2009) Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril 94(1):324–330
ACOG Committee Opinion (2004) Uterine artery embolization. Obstet Gynecol 103(2):403–404
Usadi RS, Marshburn PB (2007) The impact of uterine artery embolization on fertility and pregnancy outcome. Curr Opin Obstet Gynecol 19(3):279–283
Bradley LD (2009) Uterine fibroid embolization: a viable alternative to hysterectomy. Am J Obstet Gynecol 201(2):127–135
Katsumori T, Kasahara T, Tsuchida Y et al (2008) Amenorrhea and resumption of menstruation after uterine artery embolization for fibroids. Int J Gynaecol Obstet 103(3):217–221
Morita Y, Ito N, Hikida H et al (2007) Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids—early experience. Eur J Obstet Gynecol Reprod Biol 139(2):199–203
Stewart EA, Gostout B, Rabinovici J et al (2007) Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 110(2 Pt 1):279–287
Clevenger-Hoeft M, Syrop CH, Stovall DW et al (1999) Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol 94(4):516–520
Sulaiman S, Khaund A, McMillan N et al (2004) Uterine fibroids—do size and location determine menstrual blood loss? Eur J Obstet Gynecol Reprod Biol 115(1):85–89
Funaki K, Fukunishi H, Sawada K (2009) Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 34(5):584–589
Emanuel MH, Wamsteker K (2005) The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol 12(1):62–66
Declaration of interest
Dr. Garza is a consultant for Gynesonics and has stock options. Dr. Coad is a consultant for Gynesonics. Drs. León and Saenz have no conflicts to disclose. The remaining authors are employees of Gynesonics and have stock options. The Universidad Autónoma de Nuevo León received remuneration for patient treatment and West Virginia University received compensation for pathologic analysis of hysterectomy specimens. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10397-011-0665-9
Rights and permissions
About this article
Cite this article
Garza-Leal, J.G., Toub, D., León, I.H. et al. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate System: safety, tolerability, and ablation results in a closed abdomen setting. Gynecol Surg 8, 327–334 (2011). https://doi.org/10.1007/s10397-010-0655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10397-010-0655-3