Abstract
Purpose
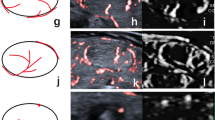
Contrast-enhanced ultrasound (CEUS) and superb microvascular imaging (SMI) can detect microvessels in thyroid nodules. Here, SMI and CEUS were compared for differentiating thyroid nodules.
Methods
Seventy-six patients (102 nodules) underwent SMI and CEUS. The SMI blood flow (BF) grade and CEUS enhanced intensity (EI) were recorded for the periphery and center of each nodule, and evaluated relative to pathological findings.
Results
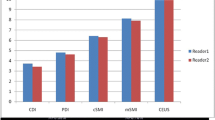
SMI of malignant nodules showed abundant BF in the periphery but lack of BF in the center, while CEUS showed hypoenhancement in the center and periphery. Large and small vessels had greater saliency on SMI-BF grade and CEUS-EI, respectively. Analysis of SMI-BF as diagnostic of thyroid carcinoma specifically at the nodule periphery indicated rates of 82.2%, 79.3%, and 81.3% for sensitivity, specificity, and accuracy, respectively. The corresponding rates for CEUS hypoenhancement were 80.8%, 86.2%, and 82.3%, respectively. Thus, SMI and CEUS rates were similar.
Conclusion
SMI-BF grade was more affected by vessels of larger inner diameter, while CEUS-EI was more affected by vessels of smaller inner diameter. SMI alone is sufficient for evaluation of blood flow in thyroid nodules, and the diagnostic value of SMI-BF of the periphery is comparable to CEUS hypoenhancement to differentiate thyroid cancer.






Similar content being viewed by others
References
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
Smith-Bindman R, Lebda P, Feldstein VA, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics. JAMA Intern Med. 2013;173:1788–96.
Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–9.
Stabenow E, Tavares MR, Ab'Saber AM, et al. Angiogenesis as an indicator of metastatic potential in papillary thyroid carcinoma. Clinics (Sao Paulo). 2005;60:233–40.
Ferrari SM, Fallahi P, Politti U, et al. Molecular targeted therapies of aggressive thyroid cancer. Front Endocrinol (Lausanne). 2015;6:176.
Baig FN, Lunenburg J, Liu S, et al. Computer-aided assessment of regional vascularity of thyroid nodules for prediction of malignancy. Sci Rep. 2017;7:14350.
Ma X, Zhang B, Ling W, et al. Contrast-enhanced sonography for the identification of benign and malignant thyroid nodules: Systematic review and meta-analysis. J Clin Ultrasound. 2016;44:199–209.
Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Skuletic V, Radosavljevic GD, Pantic J, et al. Angiogenic and lymphangiogenic profiles in histological variants of papillary thyroid carcinoma. Pol Arch Intern Med. 2017;127:429–37.
Lee JH, Shin HJ, Yoon JH, et al. Predicting lymph node metastasis in patients with papillary thyroid carcinoma by vascular index on power Doppler ultrasound. Head Neck. 2017;39:334–40.
Zhou Q, Jiang J, Shang X, et al. Correlation of contrast-enhanced ultrasonographic features with microvessel density in papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2014;15:7449–522.
Wilson NM, Masoud AM, Barsoum HB, et al. Correlation of power Doppler with microvessel density in assessing prostate needle biopsy. Clin Radiol. 2004;59:946–50.
Iared W, Shigueoka DC, Cristófoli JC, et al. Use of color Doppler ultrasonography for the prediction of malignancy in follicular thyroid neoplasms: systematic review and meta-analysis. J Ultrasound Med. 2010;29:419–25.
Hong Y, Wu Y, Luo Z, et al. Impact of nodular size on the predictive values of gray-scale, color-Doppler ultrasound, and sonoelastography for assessment of thyroid nodules. J Zhejiang Univ Science B. 2012;13:707–16.
Yang G, Fried KO. Most thyroid cancers detected by sonography lack intranodular vascularity on color Doppler imaging: review of the literature and sonographic–pathologic correlations for 698 thyroid neoplasms. J Ultrasound Med. 2017;36:89–94.
Wu MH, Chen CN, Chen KY, et al. Quantitative analysis of dynamic power Doppler sonograms for patients with thyroid nodules. Ultrasound Med Biol. 2013;39:1543–51.
Kim DW, Jung SJ, Eom JW, et al. Color Doppler features of solid, round, isoechoic thyroid nodules without malignant sonographic features: a prospective cytopathological study. Thyroid. 2013;23:472–6.
Iannuccilli JD, Cronan JJ. Monchik JM Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–64.
Zhang Y, Zhou P, Tian S, et al. Usefulness of combined use of contrast-enhanced ultrasound and TI-RADS classification for the differentiation of benign from malignant lesions of thyroid nodules. Eur Radiol. 2017;27:1527–36.
Lu R, Meng Y, Yan Z, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging. 2017;17:65.
Yongfeng Z, Ping Z, Wengang L, et al. Application of a novel microvascular imaging technique in breast lesion evaluation. Ultrasound Med Biol. 2016;42:2097–105.
Wu L, Yen H, Soon M. Spoke-wheel sign of focal nodular hyperplasia revealed by superb micro-vascular ultrasound imaging. QJM. 2015;108:669–70.
Xiao XY, Chen X, Guan XF, et al. Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol. 2016;89:20160546.
Kong J, Li JC, Wang HY, et al. Role of superb micro-vascular imaging in the preoperative evaluation of thyroid nodules: comparison with power Doppler flow imaging. J Ultrasound Med. 2017;36:1329–37.
Kim JW, Jeong YY, Chang NK, et al. Perfusion CT in colorectal cancer: comparison of perfusion parameters with tumor grade and microvessel density. Korean J Radiol. 2012;13:S89–97.
Liu JY, Yuan JP, Geng XF, et al. Morphological study and comprehensive cellular constituents of milky spots in the human omentum. Int J Clin Exp Pathol. 2015;8:12877–84.
Sancak S. Aricken H Increased percentage of microvessels but decreased density of large vessels in papillary carcinomas as compared to hot and cold thyroid nodules. Exp Clin Endocrinol Diabetes. 2009;117:637–44.
Rzeszutko M, Rzeszutko W, Dziegiel P. The morphological analysis of vasculature in thyroid tumours: immunoexpression of CD34 antigen. Folia Histochem Cytobiol. 2004;42:235–40.
De la Torre NG, Buley I, Wass JA, et al. Angiogenesis and lymphangiogenesis in thyroid proliferative lesions: relationship to type and tumour behaviour. Endocr Relat Cancer. 2006;13:931–44.
Gulubova M, Ivanova K, Ananiev J, et al. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip. 2014;28:508–17.
Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc. 2011;49:137–49.
Alexander AA, Nazarian LN, Capuzzi DM, et al. Color Doppler sonographic detection of tumor flow in superficial melanoma metastases: histologic correlation. J Ultrasound Med. 1998;17:123–6.
Forsberg F, Kuruvilla B, Pascua MB, et al. Comparing contrast-enhanced color flow imaging and pathological measures of breast lesion vascularity. Ultrasound Med Biol. 2008;34:1365–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
All procedures were conducted in accordance with the ethical standards of The Ethics Committee of The Third Xiangya Hospital of Central South University, and with the Helsinki Declaration. Informed consent was obtained from the patients.
Conflict of interest
Zhao Yongfeng, Zhou Ping, Peng Hong, Liu Wengang, and Zhang Yan declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yongfeng, Z., Ping, Z., Hong, P. et al. Superb microvascular imaging compared with contrast-enhanced ultrasound to assess microvessels in thyroid nodules. J Med Ultrasonics 47, 287–297 (2020). https://doi.org/10.1007/s10396-020-01011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-020-01011-z